
ij�о���ѧϰС����0.20mol��L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ��ʵ��̽������Ϊ���¼�����
�� ������ˮϴ�Ӽ�ʽ�ζ��ܣ�������ע��NaOH��Һ����0���̶�������
�ڹ̶��õζ��ܲ�ʹ�ζ��ܼ������Һ��
�� ����Һ������0����0���̶������£������¶���
�� ��ȡ20.00mL����Һע��ྻ����ƿ�У�������3�η�̪��Һ
�� �ñ�Һ�ζ����յ㣬���µζ���Һ�������
ʵ��������ʵŨ�Ȳ��ܴ���ָ����ʦ�����¶��ص㻷�ڽ����˷�˼������������Ҳ�������۲���ش�
��1�����ϲ����д�����ǣ����ţ� ���ô�������ᵼ�²ⶨ���(�ƫ����ƫС������Ӱ�족)__________________
��2��������У��ڼ��µζ���Һ�����ʱ���ζ��ܼ��������ݣ������²ⶨ���(�ƫ����ƫС������Ӱ�족)___________________
��3���жϵζ��յ�������ǣ���ƿ����Һ�� ɫ��Ϊ ɫ���Ұ���Ӳ���ɫ��
��4����ͼ��ij�εζ�ʱ�ĵζ����е�Һ�棬�����Ϊ mL
 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ʵ����� | ˫��ˮ��� | ���� | �������� |
| a | 15mL | �� | |
| b | 15mL | 0.5g CuO | |
| c | 15mL | 0.5g MnO2 |
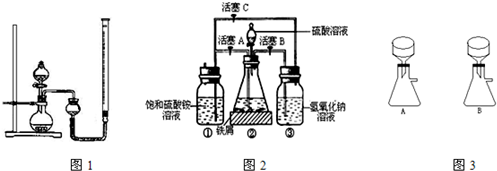
- 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�о���ѧϰС����0.20mol��L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ��ʵ��̽������Ϊ���¼�����
�� ������ˮϴ�Ӽ�ʽ�ζ��ܣ�������ע��NaOH��Һ����0���̶�������
�� �̶��õζ��ܲ�ʹ�ζ��ܼ������Һ��
�� ����Һ������0����0���̶������£������¶���
�� ��ȡ20.00mL����Һע��ྻ����ƿ�У�������3�η�̪��Һ
�� �ñ�Һ�ζ����յ㣬���µζ���Һ�������
ʵ��������ʵŨ�Ȳ��ܴ���ָ����ʦ�����¶��ص㻷�ڽ����˷�˼������������Ҳ�������۲���ش�
��1�����ϲ����д�����ǣ����ţ� ���ô�������ᵼ�²ⶨ���(�ƫ����ƫС������Ӱ�족)__________________
��2��������У��ڼ��µζ���Һ�����ʱ���ζ��ܼ��������ݣ������²ⶨ���(�ƫ����ƫС������Ӱ�족)___________________
��3���жϵζ��յ�������ǣ���ƿ����Һ�� ɫ��Ϊ ɫ���Ұ���Ӳ���ɫ��
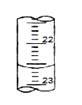
��4����ͼ��ij�εζ�ʱ�ĵζ����е�Һ�棬�����Ϊ mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
ij�о���ѧϰС����0.20mol��L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ��ʵ��̽������Ϊ���¼�����
�� ������ˮϴ�Ӽ�ʽ�ζ��ܣ�������ע��NaOH��Һ����0���̶�������
�� �̶��õζ��ܲ�ʹ�ζ��ܼ������Һ��
�� ����Һ������0����0���̶������£������¶���
�� ��ȡ20.00mL����Һע��ྻ����ƿ�У�������3�η�̪��Һ
�� �ñ�Һ�ζ����յ㣬���µζ���Һ�������
ʵ��������ʵŨ�Ȳ��ܴ���ָ����ʦ�����¶��ص㻷�ڽ����˷�˼������������Ҳ�������۲���ش�
��1�����ϲ����д�����ǣ����ţ� ���ô�������ᵼ�²ⶨ���(�ƫ����ƫС������Ӱ�족)__________________
��2��������У��ڼ��µζ���Һ�����ʱ���ζ��ܼ��������ݣ������²ⶨ���(�ƫ����ƫС������Ӱ�족)___________________
��3���жϵζ��յ�������ǣ���ƿ����Һ�� ɫ��Ϊ ɫ���Ұ���Ӳ���ɫ��
��4����ͼ��ij�εζ�ʱ�ĵζ����е�Һ�棬�����Ϊ mL

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�о���ѧϰС����0.20mol��L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ��ʵ��̽������Ϊ���¼�����
�� ������ˮϴ�Ӽ�ʽ�ζ��ܣ�������ע��NaOH��Һ����0���̶�������
�� �̶��õζ��ܲ�ʹ�ζ��ܼ������Һ��
�� ����Һ������0����0���̶������£������¶���
�� ��ȡ20.00mL����Һע��ྻ����ƿ�У�������3�η�̪��Һ
�� �ñ�Һ�ζ����յ㣬���µζ���Һ�������
ʵ��������ʵŨ�Ȳ��ܴ���ָ����ʦ�����¶��ص㻷�ڽ����˷�˼������������Ҳ�������۲���ش�
��1�����ϲ����д�����ǣ����ţ� ���ô�������ᵼ�²ⶨ���(�ƫ����ƫС������Ӱ�족)__________________
 ��2��������У��ڼ��µζ���Һ�����ʱ���ζ��ܼ��������ݣ������²ⶨ���(�ƫ����ƫС������Ӱ�족)___________________
��2��������У��ڼ��µζ���Һ�����ʱ���ζ��ܼ��������ݣ������²ⶨ���(�ƫ����ƫС������Ӱ�족)___________________
��3���жϵζ��յ�������ǣ���ƿ����Һ�� ɫ��Ϊ ɫ���Ұ���Ӳ���ɫ��
��4����ͼ��ij�εζ�ʱ�ĵζ����е�Һ�棬�����Ϊ mL
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com