
| ||
| 16m2 |
| (m1-m2) |
| 16m2 |
| (m1-m2) |


 ÖĒ»ŪæĪĢĆĆܾķ100·Öµ„ŌŖ¹ż¹Ų¼ģ²āĻµĮŠ“š°ø
ÖĒ»ŪæĪĢĆĆܾķ100·Öµ„ŌŖ¹ż¹Ų¼ģ²āĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011½ģø£½ØŹ”ĘĪĢļŹŠøßČżŹŹÓ¦ŠŌĮ·Ļ°£ØĄķ×Ū£©»Æѧ²æ·Ö ĢāŠĶ£ŗŹµŃéĢā
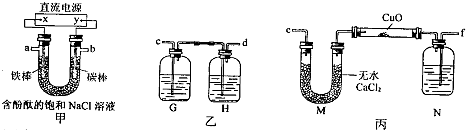
£Ø15·Ö£©Ä³»ÆѧŠĖȤŠ”×éÄā²ÉÓĆĻĀĶ¼×°ÖĆ¼×µē½ā±„ŗĶĀČ»ÆÄĘČÜŅŗ£¬²¢ÓƵē½ā²śÉśµÄH2»¹ŌCuO·ŪÄ©Ą“²ā¶ØCuµÄĻą¶ŌŌ×ÓÖŹĮæAr(Cu)£¬Ķ¬Ź±¼ģŃéĀČĘųµÄŃõ»ÆŠŌ£¬Ķ¼ÖŠ¼Š³ÖŗĶ¼ÓČČŅĒĘ÷ŅŃĀŌČ„”£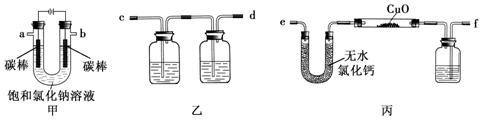
£Ø1£©Š“³ö×°ÖĆ¼×ÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ ”£
£Ø2£©ĪŖĶź³ÉÉĻŹöŹµŃé£¬ÕżČ·µÄĮ¬½ÓĖ³ŠņĪŖaĮ¬ £¬bĮ¬ £ØĢīŠ“Į¬½ÓµÄ×ÖÄø£©”£
£Ø3£©×°ÖĆŅŅÖŠµŚŅ»øö¹ćæŚĘæÄŚµÄČÜŅŗ²»ÄÜŹĒ£Ø £©
| A£®µķ·Ūµā»Æ¼ŲČÜŅŗ | B£®NaOHČÜŅŗ |
| C£®FeCl2ÓėKSCN»ģŗĻČÜŅŗ | D£®Na2SO3ČÜŅŗ |
 ¶ŌÓ²ÖŹ²£Į§¹ÜĄļµÄŃõ»ÆĶ·ŪÄ©¼ÓČČĒ°£¬Šč½ųŠŠµÄ²Ł×÷ĪŖ ”£
¶ŌÓ²ÖŹ²£Į§¹ÜĄļµÄŃõ»ÆĶ·ŪÄ©¼ÓČČĒ°£¬Šč½ųŠŠµÄ²Ł×÷ĪŖ ”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğŗÓÄĻŌ„¶«”¢Ō„±±Ź®ĖłĆūŠ£øßČż½×¶Ī²āŹŌ£ØĖÄ£©Ąķ×Ū»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø15·Ö£©ŠæĆĢøɵē³ŲĖłŗ¬µÄ¹Æ”¢Ėį»ņ¼īµČŌŚ·ĻĘśŗó½ųČė»·¾³ÖŠ½«Ōģ³ÉŃĻÖŲĪ£ŗ¦”£¶Ō·Ļ¾Éµē³Ų½ųŠŠ×ŹŌ“»Æ“¦ĄķĻŌµĆ·Ē³£ÖŲŅŖ”£Ä³»ÆѧŠĖȤŠ”×éÄā²ÉÓĆČēĻĀ“¦Ąķ·½·Ø»ŲŹÕ·Ļµē³ŲÖŠµÄø÷ÖÖ׏Ō“”£
£Ø1£©¼īŠŌŠæĆĢøɵē³ŲµÄµē½āÖŹĪŖKOH£¬×Ü·“Ó¦ĪŖZn+2MnO2+2H2O=2MnOOH+Zn(OH)2£¬Ęäøŗ¼«µÄµē¼«·“Ó¦Ź½ĪŖ_________________________________________________________”£
£Ø2£©Ģī³äĪļÓĆ60”ęĪĀĖ®Čܽā£¬ÄæµÄŹĒ¼ÓæģČܽāĖŁĀŹ£¬µ«±ŲŠėæŲÖĘĪĀ¶Č²»ÄÜĢ«øߣ¬ĘäŌŅņŹĒ___________”£
£Ø3£©²Ł×÷AµÄĆū³ĘĪŖ_____________ ”£
£Ø4£©ĀĖŌüµÄÖ÷ŅŖ³É·ÖĪŖŗ¬ĆĢ»ģŗĻĪļ£¬Ļņŗ¬ĆĢ»ģŗĻĪļÖŠ¼ÓČėŅ»¶ØĮæµÄĻ”ĮņĖį”¢Ļ”²ŻĖį£¬²¢²»¶Ļ½Į°čÖĮĪŽĘųÅŻĪŖÖ¹”£ĘäÖ÷ŅŖ·“Ó¦ĪŖ2MnO(OH)+MnO2+2H2C2O4+3H2SO4=2MnSO4+4CO2”ü+6H2O”£
¢Łµ±1 mol MnO2²Ī¼Ó·“Ó¦Ź±£¬¹²ÓŠ___________molµē×Ó·¢Éś×ŖŅĘ”£
¢ŚMnO(OH)ÓėÅØŃĪĖįŌŚ¼ÓČČĢõ¼žĻĀŅ²æÉ·¢Éś·“Ó¦£¬ŹŌŠ“³öĘä·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ________________”£
£Ø5£©ĶƱČܽāŹ±¼ÓČėH2O2µÄÄæµÄŹĒ_______________________________£ØÓĆ»Æѧ·½³ĢŹ½±ķŹ¾£©”£ĶƱČܽāĶźČ«ŗó£¬æɲÉÓĆ_____________·½·Ø³żČ„ČÜŅŗÖŠ¹żĮæµÄH2O2”£
£Ø6£©ŠæĆĢøɵē³ŲĖłŗ¬µÄ¹ÆæÉÓĆKMnO4ČÜŅŗĪüŹÕ”£ŌŚ²»Ķ¬pHĻĀ£¬KMnO4ČÜŅŗ¶ŌHgµÄĪüŹÕĀŹ¼°Ö÷ŅŖ²śĪļČēĻĀĶ¼ĖłŹ¾£ŗ
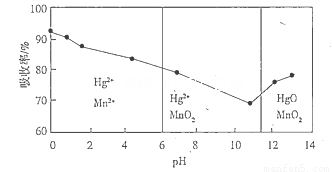
øł¾ŻÉĻĶ¼æÉÖŖ£ŗ
¢ŁpH¶ŌHgĪüŹÕĀŹµÄÓ°Ļģ¹ęĀÉŹĒ__________________________________________________.
¢ŚŌŚĒæĖįŠŌ»·¾³ĻĀHgµÄĪüŹÕĀŹøßµÄŌŅņæÉÄÜŹĒ_____________________________________.
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğÉĀĪ÷Ź”Ī÷°²ŹŠøßČżµŚŅ»“ĪÖŹ¼ģ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŹµŃéĢā
ij»ÆѧŠĖȤŠ”×éÄā²ÉÓĆĻĀĶ¼ĖłŹ¾×°ÖƵē½ā±„ŗĶĀČ»ÆÄĘČÜŅŗÖʱøH2£¬ĶعżH2»¹ŌŃõ»ÆĶ²ā¶ØCuµÄĻą¶ŌŌ×ÓÖŹĮæAr£ØCu£©£¬Ķ¬Ź±¼ģŃéCl2µÄŃõ»ÆŠŌ£ØĶ¼ÖŠ¼Š³ÖŗĶ¼ÓČČŅĒĘ÷ŅŃĀŌČ„£©”£
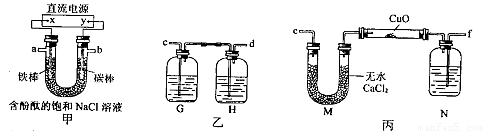
£Ø1£©Ö±Į÷µēŌ“ÖŠµÄX¼«ĪŖ ¼«£ØĢī”°Õż”±”¢”°øŗ”±”¢”°Ņõ”±»ņ”°Ńō”±£©£»Š“³ö¼××°ÖĆUŠĪ¹ÜÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ £»ŹµŃéæŖŹ¼ŗó£¬ÓĆĢś°ō×÷µē¼«µÄŅ»²ąµÄŹµŃéĻÖĻóŹĒ ”£
£Ø2£©ĪŖĶź³ÉÉĻŹöŹµŃé£¬ÕżČ·µÄĮ“½ÓĖ³ŠņĪŖ£ŗaĮ¬ £¬bĮ¬ £ØĢīŠ“Į¬½ÓµÄ×ÖÄø£©”£
£Ø3£©×°ÖĆŅŅÖŠµÄGĘæÄŚČÜŅŗæÉÄÜĪŖ £ØĢī×ÖÄø£©”£
A£®µķ·ŪKIČÜŅŗ B£®NaOHČÜŅŗ C£®Na2SČÜŅŗ D£®Na2SO3ČÜŅŗ
HĘæÄŚµÄ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗ ”£
£Ø4£©ŌŚ¶ŌÓ²ÖŹ²£Į§ŹŌ¹ÜĄļµÄŃõ»ÆĶ·ŪÄ©¼ÓČČĒ°ŠčŅŖ½ųŠŠµÄ²Ł×÷ĪŖ£ŗ ”£
£Ø5£©×°ÖƱūÖŠNĘæÄŚŹ¢·ÅµÄŹŌ¼ĮĪŖ £¬×÷ÓĆŹĒ ”£
£Ø6£©ĪŖĮĖ²ā¶ØCuµÄĻą¶ŌŌ×ÓÖŹĮ棬ijĶ¬Ń§ĶعżŹµŃé²āµĆČēŅ»ĻĀŹż¾Ż£ŗ
I£®Ńõ»ÆĶѳʷ֏ĮæĪŖm1g
¢ņ£®·“Ó¦ŗóÓ²ÖŹ²£Į§¹ÜÖŠŹ£Óą¹ĢĢåÖŹĮæĪŖm2g
¢ó£®·“Ó¦Ē°ŗóUŠĪ¹Ü¼°Ęä¹ĢĢåÖŹĮæ²īĪŖm3g
¢ō£®·“Ó¦Ē°ŗóĘæ¼°ĘäŅŗĢåÖŹĮæ²īĪŖm4g
¢ŁĒėŃ”ŌńĄķĀŪÉĻĪó²ī×īŠ”µÄŅ»×鏿¾Ż¼ĘĖćAr£ØCu£©£¬Ar£ØCu£©= ”£
¢ŚČē¹ūŃ”ÓĆĘäĖüŹż¾Ż½ųŠŠ¼ĘĖć£¬»įµ¼ÖĀAr£ØCu£© £ØĢī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°ĪŽÓ°Ļģ”±£©£¬ĄķÓÉŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010-2011ѧğø£½ØŹ”ĘĪĢļŹŠøßČżŹŹÓ¦ŠŌĮ·Ļ°£ØĄķ×Ū£©»Æѧ²æ·Ö ĢāŠĶ£ŗŹµŃéĢā
£Ø15·Ö£©Ä³»ÆѧŠĖȤŠ”×éÄā²ÉÓĆĻĀĶ¼×°ÖĆ¼×µē½ā±„ŗĶĀČ»ÆÄĘČÜŅŗ£¬²¢ÓƵē½ā²śÉśµÄH2»¹ŌCuO·ŪÄ©Ą“²ā¶ØCuµÄĻą¶ŌŌ×ÓÖŹĮæAr(Cu)£¬Ķ¬Ź±¼ģŃéĀČĘųµÄŃõ»ÆŠŌ£¬Ķ¼ÖŠ¼Š³ÖŗĶ¼ÓČČŅĒĘ÷ŅŃĀŌČ„”£
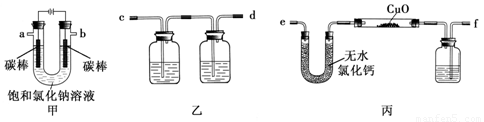
£Ø1£©Š“³ö×°ÖĆ¼×ÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ ”£
£Ø2£©ĪŖĶź³ÉÉĻŹöŹµŃé£¬ÕżČ·µÄĮ¬½ÓĖ³ŠņĪŖaĮ¬ £¬bĮ¬ £ØĢīŠ“Į¬½ÓµÄ×ÖÄø£©”£
£Ø3£©×°ÖĆŅŅÖŠµŚŅ»øö¹ćæŚĘæÄŚµÄČÜŅŗ²»ÄÜŹĒ£Ø £©
A. µķ·Ūµā»Æ¼ŲČÜŅŗ B. NaOHČÜŅŗ
C. FeCl2ÓėKSCN»ģŗĻČÜŅŗ D. Na2SO3ČÜŅŗ
£Ø4£©ŌŚ¶ŌÓ²ÖŹ²£Į§¹ÜĄļµÄŃõ»ÆĶ·ŪÄ©¼ÓČČĒ°£¬Šč½ųŠŠµÄ²Ł×÷ĪŖ ”£
£Ø5£©×°ÖƱūÖŠ¹ćæŚĘæÄŚŹ¢·ÅµÄŹŌ¼ĮĪŖ £¬×÷ÓĆŹĒ ”£
£Ø6£©ĪŖĮĖ²ā¶ØCuµÄĻą¶ŌŌ×ÓÖŹĮ棬ijĶ¬Ń§ĶعżŹµŃé²āµĆ±ū×°ÖĆ·“Ó¦Ē°ŗóČēĻĀŹż¾Ż£ŗѳʷ֏ĮæĪŖm1 g”¢·“Ó¦ŗóÓ²ÖŹ²£Į§¹ÜÖŠŹ£Óą¹ĢĢåÖŹĮæĪŖm2 g”¢·“Ó¦Ē°ŗóUŠĶ¹Ü¼°ĘäÖŠ¹ĢĢåÖŹĮæ²īĪŖm3 g”¢·“Ó¦Ē°ŗóĻ“ĘųĘæ¼°ĘäÖŠŅŗĢåÖŹĮæ²īĪŖm4 g”£
¢ŁĒėŃ”ŌńĄķĀŪÉĻĪó²ī×īŠ”µÄŅ»×鏿¾Ż¼ĘĖćAr(Cu)£¬Ar(Cu)= ”£
¢ŚČē¹ūŃ”ÓĆĘäĖū×鏿¾Ż½ųŠŠ¼ĘĖć£¬»įµ¼ÖĀAr(Cu) £ØĢī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°²»ŹÜÓ°Ļģ”±£©£¬ĄķÓÉŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com