
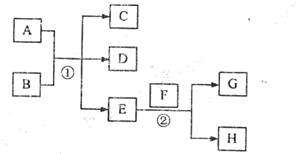
 ��C��D֮�䣬B��C��D�о����м�Ԫ�أ�д������ʱ�ٵ����ӷ���ʽ�� ��
��C��D֮�䣬B��C��D�о����м�Ԫ�أ�д������ʱ�ٵ����ӷ���ʽ�� �� ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

| T(��) | 200 | 600 | 800 |
| ��(g/L) | 6��881 | 2��650 | 1��517 |
| ����Ħ�������L/mol�� | 38��8 | 71��6 | 88��0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����aXm+ ��bYn���������ӵ��Ӳ�ṹ��ͬ����a-b=n-m |
| B��24Mg32S�����е�����������������֮��Ϊ1�U1 |
| C��CO2��PCl3�����и�ԭ������㶼����8���ӽṹ |
| D����A��Ԫ�ص��⻯���У��ȶ�����õ���е�Ҳ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
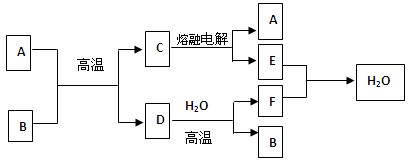
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 D ��E��F�dz��������壬����A��B��C��DΪ���ʣ��йص�ת����ϵ����ͼ��ʾ(��Ӧ����������ȥ����
D ��E��F�dz��������壬����A��B��C��DΪ���ʣ��йص�ת����ϵ����ͼ��ʾ(��Ӧ����������ȥ����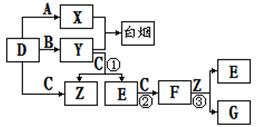
 �۵����ӷ���ʽΪ ��
�۵����ӷ���ʽΪ �� Ϊ ��
Ϊ �� ��Һ��ˮ�������c(H+)��c(OH-)�˻�Ϊ1��10-22
��Һ��ˮ�������c(H+)��c(OH-)�˻�Ϊ1��10-22�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 ��A��Ӧ��
��A��Ӧ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
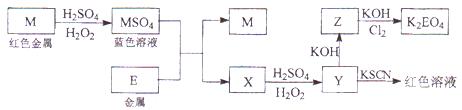
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com