
CH4��H2��C�������ʵ���Դ���ʣ�����ȼ�յ��Ȼ�ѧ����ʽΪ��
��CH4(g)��2O2(g)=CO2(g)��2H2O(l)����H����890.3 kJ��mol��1��
��2H2(g)��O2(g)=2H2O(l)����H����571.6 kJ��mol��1��
��C(s)��O2(g)=CO2(g)����H����393.5 kJ��mol��1��
(1)����д���һ�ּ���ϸ������������øʹ������O2���ò���������������ϸ��ʹ1 mol��������CO2������Һ̬ˮ���ų�������________(���������������)890.3 kJ��
(2)������CO2�����ںϳɺϳ���(��Ҫ�ɷ���һ����̼������)��CH4��CO2=2CO��2H2��1 g CH4��ȫ��Ӧ���ͷ�15.46 kJ����������
����ͼ�ܱ�ʾ�÷�Ӧ�����������仯����________(����ĸ)��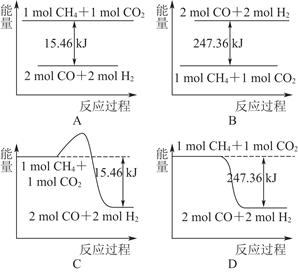
���������ʵ�����Ϊ1 mol��CH4��CO2����ij�����ܱ������У���ϵ�ų�����������ʱ��ı仯��ͼ��ʾ����CH4��ת����Ϊ________��
(3)C(s)��H2(g)����Ӧ������C(s)��2H2(g)=CH4(g)�ķ�Ӧ����ֱ�Ӳ�������ͨ��������Ӧ�������C(s)��2H2(g)=CH4(g)�ķ�Ӧ�Ȧ�H��________��
(4)Ŀǰ���������������ʵ��о���ȼ���о����ص㣬���й��������������ʵ��о������п��е���________(����ĸ)��
| A��Ѱ�����ʴ�����ʹCO2��H2O��Ӧ����CH4��O2�����ų����� |
| B��Ѱ�����ʴ������ڳ��³�ѹ��ʹCO2�ֽ�����̼��O2 |
| C��Ѱ�����ʴ���������̫����ʹ�����е�CO2�뺣���ɵ�CH4�ϳɺϳ���(CO��H2) |
| D������̬̼�ϳ�ΪC60����C60��Ϊȼ�� |
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�п�Ժ�����о����о�Ա���ʽ���������ͬ�к������Ա�������PM2.5��ѧ��ɼ���Դ�ļ��ڱ仯�о����֣�����PM2.5��6����Ҫ��Դ�����У�����β����ȼú�ֱ�ռ4%��18%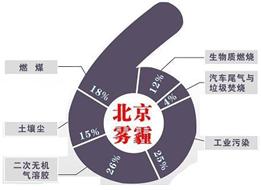
��1�����ھ�������β���ķ�ӦΪ��2NO(g)+2CO(g)
 2CO2(g)+N2(g)����֪�÷�Ӧ��570Kʱ��ƽ�ⳣ��Ϊ1��1059������Ӧ���ʼ���������˵����ȷ���ǣ�________
2CO2(g)+N2(g)����֪�÷�Ӧ��570Kʱ��ƽ�ⳣ��Ϊ1��1059������Ӧ���ʼ���������˵����ȷ���ǣ�________
| A��װ��β������װ�õ������ų��������в��ٺ���NO��CO |
| B�����β������Ч�ʵij��÷����������¶� |
| C������ѹǿ������ƽ�����ƣ���ʵ�ʲ����п�ͨ����ѹ�ķ�ʽ����侻��Ч�� |
| D�����β������Ч�ʵ����;����ʹ�ø�Ч���� |

 Ni(CO)4(g)������CO��Ӧ������������ж���Ϊ��ֹ�������ж�����ҵ�ϳ���SO2��ȥCO��������ΪS��CO2����֪��ط�Ӧ���̵������仯��ͼ��ʾ
Ni(CO)4(g)������CO��Ӧ������������ж���Ϊ��ֹ�������ж�����ҵ�ϳ���SO2��ȥCO��������ΪS��CO2����֪��ط�Ӧ���̵������仯��ͼ��ʾ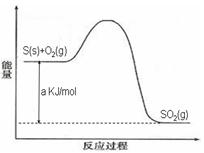
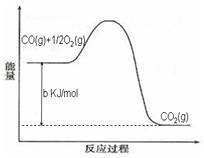
 2N2(g)+3H2O(g)��H��0��Ϊ��ߵ��������ת���ʿɲ�ȡ�Ĵ�ʩ�ǣ�������һ�֣�____________________��
2N2(g)+3H2O(g)��H��0��Ϊ��ߵ��������ת���ʿɲ�ȡ�Ĵ�ʩ�ǣ�������һ�֣�____________________��
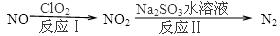
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪��������ͨ����������S8(б����)����ʽ���ڣ���������״̬ʱ������S2��S4��S6��S8�ȶ���ͬ�������壬����S4��S6��S8�������ƵĽṹ�ص㣬��ṹ����ͼ��ʾ��
��һ�������£�S8(s)��O2(g)������Ӧ����ת��ΪSO2(g)��SO3(g)����Ӧ���̺�������ϵ������ͼ��ʾ(ͼ�еĦ�H��ʾ����1 mol���������)��
��1��д����ʾS8ȼ���ȵ��Ȼ�ѧ����ʽ___________________________________��
��2��д��SO3�ֽ�����SO2��O2���Ȼ�ѧ����ʽ_______________________________________________________________��
��3����ѧ�Ϲ涨�����γ�1 mol��ѧ�����ջ�ų���������Ϊ�û�ѧ���ļ��ܣ���λkJ��mol������֪�������ļ���Ϊd kJ��mol��1���������ļ���Ϊe kJ��mol��1����S8������������ļ���Ϊ____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����֪�ڳ��³�ѹ�£���2CH3OH(l)+3O2(g)�T2CO2(g)+4H2O(g) ��H=_1275��6kJ?mol-1
��H2O(l)�TH2O(g) ��H=+44��0kJ?mol-1д����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽ�� ��
�״�������ˮ������Ӧ������������Ӧ����ʽ���£�
CH3OH(g) + H2O(g)  CO2(g) + 3H2(g) ����H>0
CO2(g) + 3H2(g) ����H>0
��1��һ�������£������Ϊ2L�ĺ����ܱ������г���1molCH3OH(g)��3molH2O(g)��20s��û�������ѹǿ�Ƿ�Ӧǰ��1��2�������ü״���ʾ�÷�Ӧ������Ϊ���� �� ��
��2���ж����п��淴Ӧ�ﵽƽ��״̬�������ǣ�����ţ��� ����
��v��(CH3OH) = 3v��(H2)�� �ڻ��������ܶȲ��䡡 �ۻ�������ƽ����Է����������� ��CH3OH��H2O��CO2��H2��Ũ�ȶ����ٷ����仯 ��CO2��H2��Ũ��֮��Ϊ1:3
��3��ͼ��P�ǿ�����ƽ�л����Ļ������ر�K������ͬ�¶�ʱ����A�����г���1molCH3OH(g)��2molH2O(g)����B�����г���1��2molCH3OH(g) ��2��4molH2O(g)���������ֱ���������Ӧ�� ��֪��ʼʱ����A��B�������ΪaL����Ӧ�ﵽƽ��ʱ����B�����Ϊ1��5aL������B��CH3OHת����Ϊ�������� ����ά�������������䣬����Kһ��ʱ������´ﵽƽ�⣬����B�����Ϊ�������� L����ͨ��������������Բ��ƣ��Ҳ������¶ȵ�Ӱ�죩��
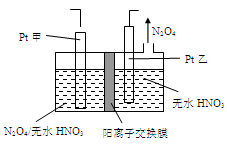
����ͼ�ס����ǵ绯ѧʵ��װ�á���ش��������⣺
��1���������о�ʢ��CuSO4��Һ
�ټ׳���ʯī���ϵĵ缫��ӦʽΪ____________________��
�������ʼʱ�ҳ�ʢ��200mL CuSO4��Һ�����һ��ʱ�����Һ��ɫ��dz����Ҫʹ��Һ�ָ������ǰ��״̬����Ҫ����Һ�м���0��8g CuO����������pHΪ ��������Һ����ı仯����
��2�����׳���ʢ�ű���NaCl��Һ����׳���ʯī���ϵĵ缫��ӦʽΪ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ú������ҵ����һ����,��������ѧ֪ʶ,�����������: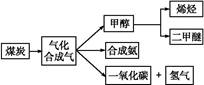
��.��֪�ò�ҵ����ij��Ӧ��ƽ�ⳣ������ʽΪ:K=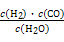 ,д��������Ӧ��Ӧ�Ļ�ѧ����ʽ:
,д��������Ӧ��Ӧ�Ļ�ѧ����ʽ:
����
��.������(CH3OCH3)��δ������������ͺ�Һ������Ϊ�ྻҺ��ȼ��ʹ�á���ҵ����CO��H2Ϊԭ������CH3OCH3����ҵ�Ʊ��������ڴ���Ӧ����(ѹ��2.0��10.0 MPa,�¶�230��280 ��)�������з�Ӧ:
��CO(g)+2H2(g) CH3OH(g)
CH3OH(g)
��H1="-90.7" kJ��mol-1
��2CH3OH(g) CH3OCH3(g)+H2O(g)
CH3OCH3(g)+H2O(g)
��H2="-23.5" kJ��mol-1
��CO(g)+H2O(g) CO2(g)+H2(g)
CO2(g)+H2(g)
��H3="-41.2" kJ��mol-1
(1)д������Ӧ����������Ӧ���ܷ�Ӧ���Ȼ�ѧ����ʽ: ��
(2)��ij�¶���,2 L�ܱ������з�����Ӧ��,��ʼʱCO��H2�����ʵ����ֱ�Ϊ2 mol��6 mol,3 min��ﵽƽ��,���CO��ת����Ϊ60%,��3 min��CO��ƽ����Ӧ����Ϊ��������������������ͬ����������ʼʱCO���ʵ���Ϊ4 mol,�ﵽƽ���CH3OHΪ2.4 mol,����ʼʱH2Ϊ��������mol��
(3)�����йط�Ӧ�۵�˵����ȷ��������������
A.������ɱ���ܱ�������,�ڷ�Ӧ�۴ﵽƽ���,����ѹ,��ƽ�ⲻ�ƶ����������ƽ����Է����������䡢��������ܶȲ���
B.��830 ��ʱ��Ӧ�۵�K=1.0,���ڴ���Ӧ���з�Ӧ�۵�K��1.0
C.ij�¶���,�����Ѵ�ƽ��ķ�Ӧ���м�������ʵ�����CO��H2O(g),��ƽ�����ơ�ƽ�ⳣ�����
(4)Ϊ��Ѱ�Һ��ʵķ�Ӧ�¶�,�о��߽�����һϵ��ʵ��,ÿ��ʵ�鱣��ԭ������ɡ�ѹǿ����Ӧʱ������ز���,ʵ������ͼ,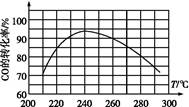
��COת�������¶ȱ仯�Ĺ����� ����
��ԭ���� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Դ�Ŀ����������������Ŀɳ�����չϢϢ��ء�
��.��֪��Fe2O3��s����3C��s��=2Fe��s����3CO��g��
��H1��a kJ��mol��1
CO��g����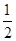 O2��g��=CO2��g������H2��b kJ��mol��1
O2��g��=CO2��g������H2��b kJ��mol��1
4Fe��s����3O2��g��=2Fe2O3��s������H3��c kJ��mol��1
��C��ȼ���Ȧ�H��________ kJ��mol��1��
��.��1������ԭ��صĹ���ԭ�������л�ѧ��Ӧ�������Ͽ�����Ƴ�ԭ��ص���________������ţ���
A��C��s����CO2��g��=2CO��g��
B��NaOH��aq����HCl��aq��=NaCl��aq����H2O��l��
C��2H2O��l��=2H2��g����O2��g��
D��2CO��g����O2��g��=2CO2��g��
�������ڵ�K2CO3��CO2Ϊ��Ӧ�Ļ�����������ѡ��Ӧ��Ƴ�һ��ԭ��أ���д����ԭ��صĸ�����Ӧ��____________________________________________________________��
��2��ijʵ��С��ģ�ҵ�ϳɰ���ӦN2��g����3H2��g�� 2NH3��g������H����92.4 kJ��mol��1����ʼ���ǽ�N2��H2�������20 mol�������1��1������5 L�ϳ����У���ӦǰѹǿΪp0����Ӧ������ѹǿ��p��ʾ����Ӧ������
2NH3��g������H����92.4 kJ��mol��1����ʼ���ǽ�N2��H2�������20 mol�������1��1������5 L�ϳ����У���ӦǰѹǿΪp0����Ӧ������ѹǿ��p��ʾ����Ӧ������ ��ʱ��t�Ĺ�ϵ��ͼ��ʾ��
��ʱ��t�Ĺ�ϵ��ͼ��ʾ��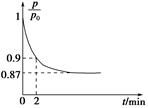
��ش��������⣺
�ٷ�Ӧ��ƽ��ı�־�ǣ�����ĸ���ţ�________��
A��ѹǿ���ֲ���
B�������ܶȱ��ֲ���
C��NH3������������N2���������ʵ�2��
��0��2 min�ڣ���c��N2���仯��ʾ��ƽ����Ӧ����Ϊ________��
�������N2��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��________��
A������ϵ�а������1��1�ٳ���N2��H2
B�������NH3
C�������¶�
D�����뺤��ʹѹǿ����
E������һ������N2
��3��25 ��ʱ��BaCO3��BaSO4���ܶȻ������ֱ���8��10��9��1��10��10��ij����BaCO3����������Һ�У�c��CO32-����0.2 mol��L��1���������������Na2SO4��Һ����Ҫ����BaSO4����������Na2SO4��Һ�����ʵ���Ũ����С��________ mol��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�й�������ŵ,��2020��,��λGDP������̼�ŷű�2005���½�40%��50%��
��1����Ч��̼���ֶ�֮һ�ǽ���,�������ⷽ������ܵ�������������
A.���ˮ����:2H2O 2H2��+O2��
2H2��+O2��
B.����ʹˮ�ֽ�����:2H2O 2H2��+O2��
2H2��+O2��
C.̫������ֽ�ˮ����:2H2O 2H2��+O2��
2H2��+O2��
D.��Ȼ������:CH4+H2O CO+3H2
CO+3H2
��2��CO2��ת�����л���ʵ��̼ѭ���������Ϊ1 L���ܱ�������,����1 mol CO2��3 mol H2,һ�������·�Ӧ:CO2��g��+3H2��g�� CH3OH��g��+H2O��g������H="-49.0" kJ/mol,���CO2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ��
CH3OH��g��+H2O��g������H="-49.0" kJ/mol,���CO2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ��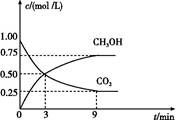
�ٴ�3 min��9 min,v��H2��=��������mol/��L��min����
����˵��������Ӧ�ﵽƽ��״̬�����������������ţ���
A.��Ӧ��CO2��CH3OH�����ʵ���Ũ��֮��Ϊ1��1����ͼ�н���㣩
B.���������ܶȲ���ʱ��ı仯���仯
C.��λʱ��������3 mol H2,ͬʱ����1 mol H2O
D.CO2����������ڻ�������б��ֲ���
��3����ҵ��,CH3OHҲ����CO��H2�ϳɡ��ο��ϳɷ�ӦCO��g��+2H2��g�� CH3OH��g����ƽ�ⳣ��:
CH3OH��g����ƽ�ⳣ��:
| �¶�/�� | 0 | 100 | 200 | 300 | 400 |
| ƽ�ⳣ�� | 667 | 13 | 1.9��1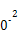 | 2.4��1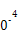 | 1��1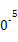 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)�״���һ����Ҫ�Ļ�����Ʒ�������ü״��������Ʊ���ȩ����ȩ����̬�״�ת����������ϵ��ͼ��ʾ��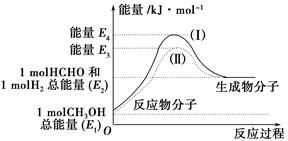
��Ӧ�����е�������ϵ
�ټ״�������ת��Ϊ��ȩ�ķ�Ӧ��________(����ȡ����ȡ�)��Ӧ��
�ڹ��̢�����̢�ķ�Ӧ���Ƿ���ͬ��____________ԭ����__________________________________��
��д���״�������ת��Ϊ��ȩ���Ȼ�ѧ��Ӧ����ʽ________________________________��
(2)��֪����CH3OH(g)��H2O(g)=CO2(g)��3H2(g)����H����49.0 kJ��mol��1
��CH3OH(g)��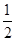 O2(g)=CO2(g)��2H2(g)����H����192.9 kJ��mol��1
O2(g)=CO2(g)��2H2(g)����H����192.9 kJ��mol��1
����˵����ȷ����________��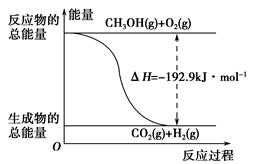
| A��CH3OHת���H2�Ĺ���һ��Ҫ�������� |
| B���ٷ�Ӧ�У���Ӧ�������������������������� |
C�����ݢ���֪��Ӧ��CH3OH(l)��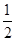 O2(g)=CO2(g)��2H2(g)�Ħ�H����192.9 kJ��mol��1 O2(g)=CO2(g)��2H2(g)�Ħ�H����192.9 kJ��mol��1 |
| D����Ӧ�ڵ������仯��ͼ��ʾ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Դ�Ŀ������������������Ŀɳ�����չϢϢ��أ�������ú���Դ�ǰ���������ǰ���ش���⡣
����֪����Fe2O3(s)��3C(ʯī)��2Fe(s)��3CO(g) ��H��a kJ��mol��1
��CO(g)��l/2O2(g)��CO2(g) ��H��b kJ��mol��1
��C(ʯī)��O2(g)��CO2(g) ��H��c kJ��mol��1
��Ӧ4Fe(s)��3O2(g)��2Fe2O3(s)���ʱ䦤H�� kJ��mol��1��
������ԭ��صĹ���ԭ�������л�ѧ��Ӧ�������Ͽ�����Ƴ�ԭ��ص��� �������)��
A��C(s)��CO2(g)��2CO(g) ��H��0 B��NaOH(aq)��HCl(aq)��NaCl(aq)��H2O(l) ��H��0
C��2H2O(l)��2H2(g)��O2(g) ��H��0 D��CH4(g)��2O2(g)��CO2(g)��2H2O(l) ��H��0
����ϡ����Ϊ�������Һ�����ԭ��ص�������ӦʽΪ ��
��������Ϊһ����ɫ��Դ����������������뷢չ����ʮ����Ҫ�����塣
��1��ʵ���ã���ͨ������£�1 g H2��ȫȼ������Һ̬ˮ���ų�142.9 kJ��������H2ȼ�յ��Ȼ�ѧ����ʽΪ ��
��2���������ϳɰ����Ȼ�ѧ����ʽΪN2(g)��3H2(g) 2NH3(g) ��H����92.4 kJ��mol��1
2NH3(g) ��H����92.4 kJ��mol��1
��һ�������£�������������˵���÷�Ӧ�Ѵ�ƽ��״̬���� ��
A������(N2)������(NH3)
B�������ʵ����ʵ������
C�������������ʵ������ٱ仯
D�����������ܶȲ��ٱ仯
����ͼ��ʾ�ϳɰ���Ӧ�ﵽƽ���ÿ��ֻ�ı��¶ȡ�ѹǿ�������е�ijһ��������Ӧ���ʦ���ʱ��t�Ĺ�ϵ�����б�ʾƽ�������е�NH3�ĺ�����ߵ�һ��ʱ���� ��ͼ��t3ʱ�ı������������ ��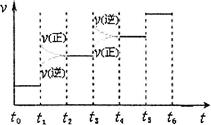
���¶�ΪT��ʱ����4a mol H2��2a mol N2����0.5 L�ܱ������У���ַ�Ӧ����N2��ת����Ϊ50%����Ӧ��ƽ�ⳣ��Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com