
��12�֣���ijһ��Ӧ��ϵ���з�Ӧ��������ﹲ5�����ʣ�S��H2S��HNO3��NO��H2O���÷�Ӧ�л�ԭ������____������Ӧ������ת����0.3moL���ӣ������������������__ g��
�ƽ�a mol Cl2 ͨ�뺬b mol FeBr2����Һ�У�
��0< a / b��1/2 ʱ����Ӧ�����ӷ���ʽΪ�� 2Fe2+ + Cl2 = 2Fe3+ + 2Cl- ��
д����2�����ܷ��������ӷ���ʽ��
�� �� a / b =1ʱ��_______________________ ______;
�� ��a / b��3/2ʱ��________________ _____________��
�ǹ۲����·�Ӧ���ܽ���ɣ�Ȼ������������⣺
��
Al(OH)3 ��H2O Al(OH)4- + H+
��NH3+H2O
Al(OH)4- + H+
��NH3+H2O NH4+
+ OH_
NH4+
+ OH_
����֪B(OH)3��һԪ���ᣬ��д������뷽��ʽ________________________ __
��N2H4�Ƕ�Ԫ�����д����ڶ������뷽��ʽ___________ _________________

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
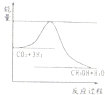
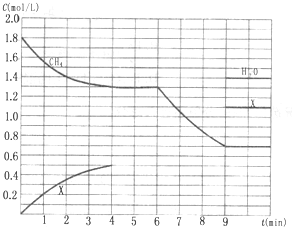 ��Ӧ��ϵ��ͬʱͨ����顢������ˮ��������������Ҫ��ѧ��Ӧ�У�
��Ӧ��ϵ��ͬʱͨ����顢������ˮ��������������Ҫ��ѧ��Ӧ�У��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ��ʼŨ�� | �� | �� | �� |
| c��H2��/mol/L | 0.010 | 0.020 | 0.020 |
| c��CO2��/mol/L | 0.010 | 0.010 | 0.020 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ũ�ȷֱ�Ϊ1 mol/L��FeCl3��FeCl2��CuCl2�����Һ100 mL������һ���������ۣ������������ա�
��1����ַ�Ӧ�������Һ�л���һ������Cu2��������Һ��һ�����еĽ������ӻ���________________������������Һ�е����ʵ�����Χ____________________��
���ܺ��еĽ������������Ϊ____________��
��2����Ӧ��Ϻ�������ʣ�࣬��Һ��һ�����еĽ�������Ϊ___________��Ϊ______mol��һ��û�еĽ�������Ϊ______________��
��3������FeCl3��Һ�м�����������ᣬ������Ӧ�����ӷ���ʽΪ____________________��
��4��ijһ��Ӧ��ϵ���з�Ӧ��������ﹲ�������ʣ�S��H2S��HNO3��NO��H2O���÷����� Ӧ�Ļ�ѧ����ʽΪ________________________________������Ӧ������ת����0.3mol���� ���ӣ������������������___________g�����ɵ������ڱ���µ������________L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�����и������ϣ����л�ѧ�Ծ��������棩 ���ͣ������
| ��ʼŨ�� | �� | �� | �� |
| c��H2��/mo1/L | 0.010 | 0.020 | 0.020 |
| c��CO2��/moI/L | 0.010 | 0.010 | 0.020 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ�߿���ѧģ���Ծ��������������棩 ���ͣ������
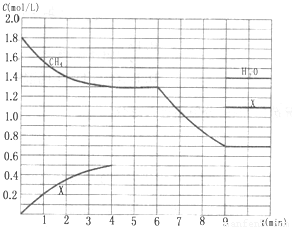
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com