
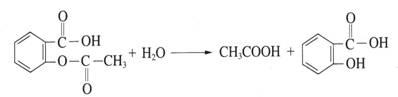
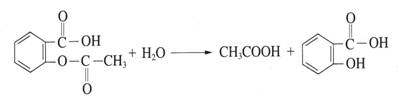
������������ʹ�����������������������һ����Ҫ��־��
��1��������Ʒ�еĶ�����Ƥ�����������Ͷ�п��Ƥ������Ƥ���Ʋ㲿���ƻ����ڳ�ʪ�Ļ����У� ���������������Ƥ����������ʴ��
��2�����dz����������������ȷ�Ӧ�����Ӹֹ죬д�������ȷ�Ӧ�Ļ�ѧ����ʽ��
��
��3�������˵�����δ��ʱϴ������Һ�к�NaCl�����ڶ�������ʴ���ֺ��ɫ��ߡ�
�������������ĸ�ʴ��Ҫ�� ��ʴ��
��������ʴ��������ӦʽΪ ��
��4��Ϊ��ֹ�ִ��Ĵ����ں�ˮ�и�ʴ��һ�����ִ�����װ��һ�������� ���п����ͭ�����顣
��5����ҵ����ԭ������Ҫ��ѧ��Ӧ����ʽ������������������Ϊ ��
(1)������
��2��
��3������ 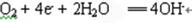
��4��п
��5��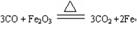
��������
�����������1���������������ã�����Ƥ��п���ã����ڳ�ʪ�Ļ����У�������������ʴ��
��2�������������ڼ��������·�Ӧ�ķ���ʽΪ ��
��
��3�����������Ի�������Ҫ��������������ʴ��
�������õ����ӣ����ϼ۽��ͣ���ӦʽΪ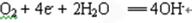 ��
��
��4��п�������ã�ͭ���������ã���ѡ��п�顣
��5����ҵ����ԭ������CO���»�ԭ������������ʽΪ ��
��
���㣺������ʴ������������� �������˳����Ӧ��
�����������������ڿ�������ѧ�������������������������ʹѧ�����ܵ���������л�ѧ������Ҳ���п�������֮һ��


 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����ĩ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��.�����г���ҩƷ�ܶ࣬�磺�ٵ�ơ�����ù�ء��۰�˾ƥ�֡���������ע��Һ���ݿ���ҩ����Ҫ�ɷ�Ϊ̼�����ƣ���
���������������ڿ����ص��� ����д��ţ�
��̼�����ƿɷ���θ����ڹ��࣬�䷢�ӹ�Чʱ�����ӷ���ʽΪ������ ������ ������
�����ǻ�������ĽṹʽΪ 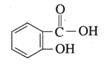 ������Ϊˮ���ᣬ��ҽѧ�Ͼ��н�����ʹ�Ϳ��ס�����ʪ���ã��������������Զ��̼�θ�����������صķ�Ӧ�����ͨ��������ת��Ϊ����ˮ���ᣨ��˾ƥ�֣������ú��������ڻỺ����Ӧ����ˮ���
������Ϊˮ���ᣬ��ҽѧ�Ͼ��н�����ʹ�Ϳ��ס�����ʪ���ã��������������Զ��̼�θ�����������صķ�Ӧ�����ͨ��������ת��Ϊ����ˮ���ᣨ��˾ƥ�֣������ú��������ڻỺ����Ӧ����ˮ���
�ݴˣ���ش�
�����Ӧ�ķ�Ӧ����Ϊ ��
��ʱ���������ҽ�����ˮ�����Ƴ������λ���Σ�Ŀ���� ���Է�ֹ�̼�θ�Ĥ��
(4)���й���ҩ��ʹ�õ�˵���У���ȷ����__________������д��ţ�
A�������ʹ�����ʱ��ԣ��������������
B�����ڴ������ð�˾ƥ�ֿ�Ԥ��������û�ж�������
C��ʹ����ù�ؿ�ֱ�Ӿ���ע�䣬�������Ƥ����������
D������ƽ��ҩ���Ŀ��裬�����˶����Ե�ҩ���Լ���ҩ���ã����õ�ҽԺ����
��.������������Ҫ����ҪӪ���أ���������Ҫ��һ���ǡ�
���Թ��м���0.5g���ۺ�4mL20%��H2SO4��Һ������3��4min��Ȼ���ü�Һ�к��Թ��е�H2SO4��Һ����ش��������⣺
��1���������ʸ������۵��� ������ס�����ˡ������⡱��
��2��������ȫˮ�������л���Ļ�ѧʽΪ ������ˮ����������ڱ������Ļ�ѧ����ʽΪ___________________________________��
��3����Ҫ�������û����ȫˮ�⣬��ȡ����������Һ���� �����Լ������ƣ���Ӧ�۲쵽 ��
��.��ýྻ��ȫ������ˮ��ÿ���˵�������Ҫ��ijũ�����Ϊ�������ˮ���ڽ��ر�ˮȡ�ؼҺ�ʹ��Ư�ۻ�Ư��Ƭ����ɱ����������ԭ�����û�ѧ����ʽ��ʾΪ ��
��.������������ʹ�����������������������һ����Ҫ��־��
��1����ҵ��������Ҫ��ѧ��Ӧ����ʽΪ ��
��2�������˵�����δ��ʱϴ������Һ�к�NaCl��,�ڶ�������ʴ���ֺ��ɫ��ߡ��Իش�
�������ĸ�ʴ��Ҫ���� ��ʴ��ɵġ�
��������ʴ�ĸ�����ӦʽΪ ��
��3��Ϊ��ֹ�ִ��Ĵ����ں�ˮ�и�ʴ��һ���ڴ������� ���п�顱��ͭ�顱����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��.�����г���ҩƷ�ܶ࣬�磺�ٵ�ơ�����ù�ء��۰�˾ƥ�֡���������ע��Һ���ݿ���ҩ����Ҫ�ɷ�Ϊ̼�����ƣ���
���������������ڿ����ص��� ����д��ţ�
��̼�����ƿɷ���θ����ڹ��࣬�䷢�ӹ�Чʱ�����ӷ���ʽΪ������ ������ ������
�����ǻ�������ĽṹʽΪ 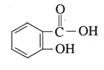 ������Ϊˮ���ᣬ��ҽѧ�Ͼ��н�����ʹ�Ϳ��ס�����ʪ���ã��������������Զ��̼�θ�����������صķ�Ӧ�����ͨ��������ת��Ϊ����ˮ���ᣨ��˾ƥ�֣������ú��������ڻỺ����Ӧ����ˮ���
������Ϊˮ���ᣬ��ҽѧ�Ͼ��н�����ʹ�Ϳ��ס�����ʪ���ã��������������Զ��̼�θ�����������صķ�Ӧ�����ͨ��������ת��Ϊ����ˮ���ᣨ��˾ƥ�֣������ú��������ڻỺ����Ӧ����ˮ���
�ݴˣ���ش�
�����Ӧ�ķ�Ӧ����Ϊ ��
��ʱ���������ҽ�����ˮ�����Ƴ������λ���Σ�Ŀ���� ���Է�ֹ�̼�θ�Ĥ��
(4)���й���ҩ��ʹ�õ�˵���У���ȷ����__________������д��ţ�
A�������ʹ�����ʱ��ԣ��������������
B�����ڴ������ð�˾ƥ�ֿ�Ԥ��������û�ж�������
C��ʹ����ù�ؿ�ֱ�Ӿ���ע�䣬�������Ƥ����������
D������ƽ��ҩ���Ŀ��裬�����˶����Ե�ҩ���Լ���ҩ���ã����õ�ҽԺ����
��.������������Ҫ����ҪӪ���أ���������Ҫ��һ���ǡ�
���Թ��м���0.5g���ۺ�4mL20%��H2SO4��Һ������3��4min��Ȼ���ü�Һ�к��Թ��е�H2SO4��Һ����ش��������⣺
��1���������ʸ������۵��� ������ס�����ˡ������⡱��
��2��������ȫˮ�������л���Ļ�ѧʽΪ ������ˮ����������ڱ������Ļ�ѧ����ʽΪ___________________________________��
��3����Ҫ�������û����ȫˮ�⣬��ȡ����������Һ���� �����Լ������ƣ���Ӧ�۲쵽 ��
��.��ýྻ��ȫ������ˮ��ÿ���˵�������Ҫ��ijũ�����Ϊ�������ˮ���ڽ��ر�ˮȡ�ؼҺ�ʹ��Ư�ۻ�Ư��Ƭ����ɱ����������ԭ�����û�ѧ����ʽ��ʾΪ ��
��.������������ʹ�����������������������һ����Ҫ��־��
��1����ҵ��������Ҫ��ѧ��Ӧ����ʽΪ ��
��2�������˵�����δ��ʱϴ������Һ�к�NaCl��,�ڶ�������ʴ���ֺ��ɫ��ߡ��Իش𣺢������ĸ�ʴ��Ҫ���� ��ʴ��ɵġ�
��������ʴ�ĸ�����ӦʽΪ ��
��3��Ϊ��ֹ�ִ��Ĵ����ں�ˮ�и�ʴ��һ���ڴ������� ���п�顱��ͭ�顱����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com