
| �ζ����� | ������Һ�����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 0.20 | 20.20 |
���� ��1���ٵζ�����ҪҪNaOH��Һ��ϴ������ᵼ����ҺŨ��ƫ�ͣ����ƫ��
����ƿ���Ƿ���ˮ�����ʵ����ʵ������䣻
�۵ζ�ʱ�۾�Ӧע��ע��۲���ɫ�仯����ȷ���յ㣻
�ܸ��ݷ�̪�ı�ɫ��Χȷ���ζ��յ�ʱ��ɫ�仯��
��2������Һ�����Ӧȡ����ʵ���ƽ��ֵ���������Һ��H+�����ʵ��������ݷ���ʽ��֪��CH2��6N4H+�����ʵ���������ȷ����Ʒ�е�������������
��� �⣺��1���ټ�ʽ�ζ���������ˮϴ�Ӻ�ֱ�Ӽ���NaOH����Һ���еζ���NaOH��Һ��ϡ�ͣ����V������ƫ�ߣ�c��H+��ƫ�����Ʒ�е�����������Ҳ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
����ƿ������ˮϴ�Ӻ���Ȼˮδ������������Һ�е�H+�����ʵ������䣬��ζ�ʱ����NaOH����Һ�е��������Ƶ����ʵ����Ͳ��䣬��Һ��������䣬
�ʴ�Ϊ����Ӱ�죻
�۶�ʱ�ߵα�ҡ����ƿ���۾�Ӧע��۲���ɫ�仯��ȷ���ζ��յ㣻
�ʴ�Ϊ��B��
�ܴ���ҺΪ���ԣ���̪ӦΪ��ɫ������ҺתΪ����ʱ����Һ��ɫ��Ϊdz�죬���Եζ��յ�ʱ����Һ����ɫ��Ϊdz��ɫ��30s�ڲ���ɫ��
�ʴ�Ϊ���ޣ�dz�죻
��2������ʵ�����ĵı���Һ������ֱ�Ϊ��20.01mL��19.99mL��20.00mL������Ч������ʵ���ƽ��ֵΪ$\frac{20.01+19.99+20.00}{3}$mL=20.00mL��
�����������Լ�ȩ��Һһ���ǹ����ģ�����1.500g ��ξ��ܽ��ȡ������$\frac{1}{10}$���еζ�����0.15g������Һ�к���H+������CH2��6N4H+����0.02L����0.1010mol/L=0.00202mol������4NH4++6HCHO�T3H++6H2O+��CH2��6N4H+��ÿ����4molH+������CH2��6N4H+����������NH4+4mol�����Թ�����NH4+0.00202mol�����к���Ԫ��0.00202mol��14g/mol=0.02828g�����Ե�����������Ϊ$\frac{0.02828}{0.15}$��100%=18.85%��
�ʴ�Ϊ��18.85%��
���� ���⿼�����ʵĺ����IJⶨ���������к͵ζ�ԭ���Ŀ��飬ע����ѧ��ʵ�����������������ͼ����������ۺϿ��飬��Ŀ�Ѷ��еȣ�


 ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
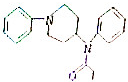 ���й��ڷ�̫ū��˵����ȷ���ǣ�������
���й��ڷ�̫ū��˵����ȷ���ǣ�������| A�� | ��̫ū�ķ���ʽΪC20H23N2O | |
| B�� | �÷���������ԭ�ӿ��Թ�ƽ�� | |
| C�� | ��̫ū���ڱ���ͬϵ�� | |
| D�� | ��̫ū��һ�������¿ɷ���ˮ�ⷴӦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�츣��ʡ�����ϵڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��X����Һ�У�����Y�Լ��������ij���ʾ��ͼ��ͼ��ʾ���ε���(ע���߶ε�б��)( )
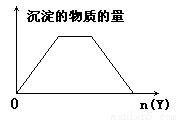
A����NaOH��Ca(OH)2�Ļ��Һ��ͨ��CO2
B����HCl��AlCl3�Ļ��Һ�еμ�NaOH
C����NH4Cl��AlCl3�Ļ��Һ�еμ�NaOH
D����NaOH��NaAlO2��Һ�еμ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�츣��ʡ�����ϵڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���й��ڽ����Ƶ������������( )
A���ƵĻ�ԭ�Ժ�ǿ,ֻ���Ի���̬��������Ȼ��
B�����ʵ�����,����С���и�,������ú����
C����ʧ�����ô���ˮ����ĭ���������
D���Ƶ���ɫ��ӦΪ��ɫ,������������ָʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �ζ����� | ������Һ���/ml[ | ��NaOH��Һ���������ml�� | |
| �ζ�ǰ/ml | �ζ���/ml | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 2.30 | 22.30 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ZԪ�������ڱ��е�λ��Ϊ�������ڵڢ���A���壬�ܷ�������ķ�Ӧ֤��Z�ķǽ����Ա�̼ǿ��2HCl+Na2CO3=NaCl+H2O+CO2������ܡ���
��ZԪ�������ڱ��е�λ��Ϊ�������ڵڢ���A���壬�ܷ�������ķ�Ӧ֤��Z�ķǽ����Ա�̼ǿ��2HCl+Na2CO3=NaCl+H2O+CO2������ܡ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
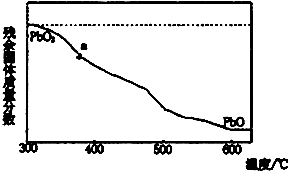 ��1��ijС�����������Ǧ�ֽ�������ɣ�ȡ478g��PbO2���ȣ�PbO2�ڼ��ȹ��̷����ֽ��ʧ��������ͼ��ʾ����֪ʧ�������ϵ�aΪ96.66%����a�����ķ���ʽΪPb2O3
��1��ijС�����������Ǧ�ֽ�������ɣ�ȡ478g��PbO2���ȣ�PbO2�ڼ��ȹ��̷����ֽ��ʧ��������ͼ��ʾ����֪ʧ�������ϵ�aΪ96.66%����a�����ķ���ʽΪPb2O3�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com