
���� ����һ����������������Һ��Ӧ����ƫ��������������
��1��þ������������Сʱ�������������������Ҫ������������Һ��࣬ʵ����Ҫ����������Һ�����Ӧ���ڻ�������ֵ���ݴ˼��㣻
��2��þ�ϻḽ��ƫ�����Ƶ����ʣ�δϴ�ӵ��²ⶨ��þ������ƫ��
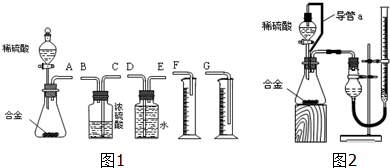
����������1����װ�õ���װ˳�Ͻ���ˮ��Ӧ������ˮ�������ⶨ���������������ʢˮ���Լ�ƿ����һ��Ҫ�̽�����������ѹǿԭ����ˮ�ų�����Ͳ��ˮ��������������������������Ͳ�ڵ���Ӧ������Ͳ�ײ���
�ڷ�Ӧ���ȵ����������¶�ƫ�ߣ���Ӧ��ȴ���ٽ��ж�ȡ�������������ȡʵ�����������������ʱ�����ƶ���Ͳ��ʹ����Һ������ƿ��Һ����ƽ�������밼Һ�����͵�ˮƽ��ȡ�����������
��2���ٱ��ַ�Һ©��������ѹǿ����ƿ������ѹǿ��ȣ���Һ©������ʱϡ������˳�����£�������ƿ��ϡ����������ڽ����Һ©��������������Ӷ��������ڼ���ϡ������������������
�ڵζ��ܵ���ֵ��̶����Ϸ������ε����֮��Ϊ�ⶨ�������������ע��Ӧ���ָ������ζ�����Һ��ȸߣ����ռ�������ζ�����Һ��������������С��
��� �⣺����һ����������������Һ��Ӧ����ƫ����������������Ӧ����ʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2����
�ʴ�Ϊ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��1����þΪ3%ʱ���������ĺ�����ߣ�5.4g�Ͻ�����������Ϊ��5.4g����1-3%��=5.4��97%g����
2Al+2NaOH+2H2O=2NaAlO2+3H2��
54g 2mol
5.4g��97%g V��10-3L��2.0mol/L
����54g����5.4g��97%g��=2mol����V��10-3L��2.0mol/L������ã�V=97����V��NaOH��Һ����97mL��
�ʴ�Ϊ��97mL��
��2��þ�ϻḽ��ƫ�����Ƶ����ʣ�δϴ�ӵ��²ⶨ��þ������ƫ��þ����������ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
����������1����װ�õ���װ˳�Ͻ���ˮ��Ӧ������ˮ�������ⶨ���������������ʢˮ���Լ�ƿ����һ��Ҫ�̽���������������ѹǿԭ����ˮ�ų�����Ͳ��ˮ��������������������������Ͳ�ڵ���Ӧ������Ͳ�ײ���������˳��Ϊ����A���ӣ�E����D���ӣ�G����
�ʴ�Ϊ��E��D��G��
�ڷ�Ӧ���ȵ����������¶�ƫ�ߣ���Ӧ��ȴ���ٽ��ж�ȡ�������������ȡʵ�����������������ʱ�����ƶ���Ͳ��ʹ����Һ������ƿ��Һ����ƽ�������밼Һ�����͵�ˮƽ��ȡ�����������
��ѡACD��
��2����װ���е���a�������ǣ����ַ�Һ©��������ѹǿ����ƿ������ѹǿ��ȣ���Һ©������ʱϡ������˳�����£�������ƿ��ϡ����������ڽ����Һ©��������������Ӷ��������ڼ���ϡ������������������
�ʴ�Ϊ��������ƿ��ϡ����������ڽ����Һ©��������������Ӷ��������ڼ���ϡ������������������
�ڵζ��ܵ���ֵ��̶����Ϸ������ε����֮��Ϊ�ⶨ��������������ռ�������ζ�����Һ�������С�����Բⶨ���������ΪV1-V2��
�ʴ�Ϊ��V1-V2��
���� ���⿼�����ʺ����IJⶨ����ʵ��ԭ����װ�õ����⡢ʵ�鷽����Ƶȣ��Ѷ��еȣ�����ʵ��ԭ���ǽ���Ĺؼ����Ƕ�֪ʶ���ۺϿ��飬��Ҫѧ������֪ʶ�Ļ������ۺ�����֪ʶ�������⡢��������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڳ��³�ѹ�£�11.2 L Cl2���еķ�����Ϊ0.5NA | |
| B�� | �ڳ��³�ѹ�£�1 mol�������еķ�����ΪNA | |
| C�� | �ڳ��³�ѹ�£�32 g������ԭ����ΪNA | |
| D�� | ��״���£�1molˮ�����ԼΪ22.4L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ������أ�K2FeO4�����и�Ч���������ã�Ϊһ�����ͷ��ȸ�Ч����������ⷨ�Ʊ�������ز�����㣬���ʸߣ�����ʵ�����Ʊ�����ԭ����ͼ��ʾ�����ܵ�ⷴӦΪ��
������أ�K2FeO4�����и�Ч���������ã�Ϊһ�����ͷ��ȸ�Ч����������ⷨ�Ʊ�������ز�����㣬���ʸߣ�����ʵ�����Ʊ�����ԭ����ͼ��ʾ�����ܵ�ⷴӦΪ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 10g 46%���Ҵ���Һ������ԭ����Ϊ1.2NA | |
| B�� | 0.5 mol���ڵ�NaHSO4�к��е�������ĿΪ1.5NA | |
| C�� | ��״���£�2.24L�״��к���C-H������ĿΪ0.3NA | |
| D�� | S2��S8�Ļ���ﹲ38.4g������������ԭ����Ϊ1.4NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ���ڹ�ҵ�ϣ����Ȼ���Ϊԭ�ϣ��ڼ�����Һ�У�ͨ�����ķ������Ƶ�NaClO�������ӷ���ʽ��ʾ��ȡNaClO�ĵ���ܷ�Ӧ��Cl-+H2O$\frac{\underline{\;ͨ��\;}}{\;}$ClO-+H2��������Ũ�ȵ������NaClO��Na2SO3��Һ��Ϻ�����ǡ����ȫ��Ӧ��д����Ϲ��̵����ӷ�Ӧ����ʽClO-+SO32-=Cl-+SO42-��
���ڹ�ҵ�ϣ����Ȼ���Ϊԭ�ϣ��ڼ�����Һ�У�ͨ�����ķ������Ƶ�NaClO�������ӷ���ʽ��ʾ��ȡNaClO�ĵ���ܷ�Ӧ��Cl-+H2O$\frac{\underline{\;ͨ��\;}}{\;}$ClO-+H2��������Ũ�ȵ������NaClO��Na2SO3��Һ��Ϻ�����ǡ����ȫ��Ӧ��д����Ϲ��̵����ӷ�Ӧ����ʽClO-+SO32-=Cl-+SO42-���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
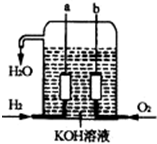 ��������������ɴ���ʹ�õ�����ȼ�ϵ����һ�����͵Ļ�ѧ��أ��乹����ͼ��ʾ�������缫���ɶ����̼�Ƴɣ�ͨ��������ɿ�϶���ݳ������ڵ缫����ŵ磮
��������������ɴ���ʹ�õ�����ȼ�ϵ����һ�����͵Ļ�ѧ��أ��乹����ͼ��ʾ�������缫���ɶ����̼�Ƴɣ�ͨ��������ɿ�϶���ݳ������ڵ缫����ŵ磮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����Ŀǰ��ֱ�����õ����������ɻ�ѧ��Ӧ������ | |
| B�� | ��ѧ�仯�е������仯��Ҫ���ɻ�ѧ���仯����� | |
| C�� | ��ѧ��Ӧ�������仯�Ĵ�С�뷴Ӧ������������� | |
| D�� | �����仯�ǻ�ѧ��Ӧ�Ļ�������֮һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
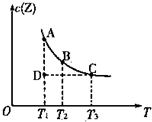 ���ݻ�������ܱ��������з�Ӧ��X��g��+Y��g��?Z��g������Z��g�������ʵ���Ũ��c��Z�����¶�T�Ĺ�ϵ��ͼ��ʾ�������ϵ�����һ�㶼��ʾƽ��״̬����������˵������ȷ���ǣ�������
���ݻ�������ܱ��������з�Ӧ��X��g��+Y��g��?Z��g������Z��g�������ʵ���Ũ��c��Z�����¶�T�Ĺ�ϵ��ͼ��ʾ�������ϵ�����һ�㶼��ʾƽ��״̬����������˵������ȷ���ǣ�������| A�� | A����B����ȣ�B���c��X���� | B�� | A����C��Ļ�ѧ��Ӧ���ʣ�A��C | ||
| C�� | �ڷ�Ӧ���е�D��ʱ��v����v�� | D�� | �÷�Ӧ������Ӧ�����ȷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 16.8L | B�� | 14L | C�� | 19.6L | D�� | 18.4L |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com