
��1��д��������C�Ļ�ѧʽ������ʽ��__________��
��2����ѧ��ͨ����ԭ�����͵���������ͬ�ķ��ӻ����ӳ�Ϊ�ȵ����塣������D�ͻ�����C�ǵȵ����塣������D��ƽ���ͷ��ӣ�Ҳ������Ԫ����ɣ��dz������ʡ�д��D�ķ���ʽ____________��
��3���ȵ�����������ƵĽṹ�����ʡ���д��C�Ľṹʽ___________���ж����й��ڻ�����C��˵����ȷ����___________����д��ĸ����
A.C�����и�ԭ�Ӳ���ͬһƽ����
B.C����ʹKMnO4������Һ��ɫ
C.C����HCl��Br2�����ӳɷ�Ӧ
D.C�����
��4��C�Ķ���ȡ�������___________��ͬ���칹�塣
(1)B3N3H6
(2)C6H6

(4)4
������(1)����B����Ԫ������֮��Ϊ14��3�Լ�B���ӽṹΪ�����Σ����ж�BΪ����NH3��������֪C�бغ���Ԫ�ء���C����Է�������Ϊ40.5��2=81,��֪��Ԫ�غ���Ԫ�ص����������ֱ���40%��7.4%����Ԫ�ص���������Ϊ1��40��-7.4�����ɼ����C�ķ���ʽ��n(B)��n(N)��n(H)=![]() =1��1��2,��C��ʵ��ʽΪBNH2���֣�BNH2��n=81�����n=3����C�ķ���ʽΪ��B3N3H6��
=1��1��2,��C��ʵ��ʽΪBNH2���֣�BNH2��n=81�����n=3����C�ķ���ʽΪ��B3N3H6��
��2���������Ϣ�����ƶϱ���C6H6����B3N3H6���ڻ�ѧ�ϱ����������������ǵȵ����壨����12��ԭ�ӣ�42�����ӣ�����DΪ���������ʽΪC6H6��
��3��������뱽����д��B3N3H6�Ľṹʽ����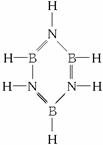

�ɴ˿��жϳ��й����Ľṹ���ʵ��ĸ�ѡ���У�ֻ��B��C����ȷ��
��4��������뱽���ɷ�����ͬ���Ķ���ȡ�������ڶ��ȱ�������ȱ����Զ��ȱ�����ͬ���칹�壬���������ġ������ȡ����ַ�Ϊ��������䡱�͡�������䡱�����������Ķ���ȡ���ﹲ��4��ͬ���칹�塣


 ��ҵ����ϵ�д�
��ҵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ������ | K+��Na+��Ba2+��NH4+ |
| ������ | CH3COO-��Cl-��OH-��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
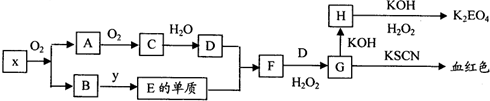
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



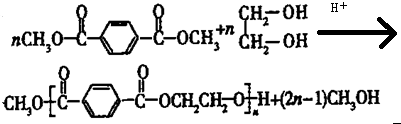
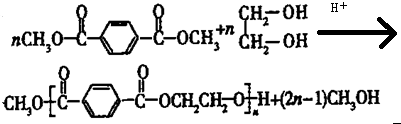
| H+ |
 �ϳ�
�ϳ� ������ͼ����ע����Ӧ������
������ͼ����ע����Ӧ�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ������ | Na+��Ba2+��NH4+ |
| ������ | CH3COO-��Cl-��OH-��SO42- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com