
����Ŀ��ij��θҩ����Ч�ɷ�Ϊ̼��ƣ��ⶨ����̼��ƺ����IJ������£����ҩƬ�е������ɷֲ���������������Ʒ�Ӧ����
������1.60mol/Lϡ�����1.60mol/LNaOH��Һ��
����һ��������ҩƬ��1.60g���м���20.00mL����ˮ��
����1.60mol/LNaOH��Һ�к�����ϡ���ᣬ��¼�����ĵ�NaOH��Һ�������
�ܼ���25.00mL1.60mol/Lϡ���ᡣ
��ش��������⣺
��1����ȷ�IJ���˳����__________________________������ţ�
��2���ⶨ�����з�����Ӧ�����ӷ���ʽΪ___________________________________��___________________________��
��3���òⶨʵ�鹲������4�Ρ�4�βⶨ�����ĵ�NaOH��Һ��������£�
�ⶨ���� | ��1�� | ��2�� | ��3�� | ��4�� |
V��NaOH(aq)��/mL | 13.00 | 12.90 | 13.10 | 13.00 |
ʵ��������50mL��100mL��250mL��500mL4�ֹ�������ƿ��������NaOH��ҺӦѡ�õ�����ƿ�Ĺ��Ϊ___________��
��4������������NaOH��Һʱ��Ҫ����Ҫ������������ƽ���ձ���________________��
�ڸ�ʵ��ĵ�һ���Ǽ���,��ȡ_____gNaOH���塣
�����ձ���ȡNaOH����ʱ,���в����������ȷ˳����__________________(��ͬ�IJ������ظ�ʹ��)��
a.������ƽ��� b.������Ż������ c.�����벦����̶ȴ�
d.��ȡС�ձ������� e.��ȡС�ձ���NaOH������ f.��¼����������
�ܳ�ȡNaOH����ʱ,�������������ƽ����,��NaOH�����������,������1.4�Ŀ̶��ߴ�����ʵ�ʳƵõ�NaOH����Ϊ____________g��
��д�����в����������Ƶ���ҺŨ����ɵ�Ӱ��(�ƫ��ƫС�����䡱)��
a.����ʱ������NaOH������������λ�õߵ���______________��
b.δϴ���ձ�����������______________��
c.����ҡ�Ⱥ���Һ����ڿ̶��ߣ����¼�ˮ���̶��ߣ�______________��
��ȡ50mL��������ȷ��NaOH��Һ����ˮϡ����100mL������NaOH��Һ�����ʵ���Ũ��Ϊ________________��
���𰸡��٢ڢܢۻ�ڢ٢ܢ� ![]()
![]() 100mL ������ƿ(100mL)����Ͳ��ҩ�ס�����������ͷ�ι� 6.4 adfefbc 3.6 a.ƫС b.ƫС c.ƫС 0.80mol/L
100mL ������ƿ(100mL)����Ͳ��ҩ�ס�����������ͷ�ι� 6.4 adfefbc 3.6 a.ƫС b.ƫС c.ƫС 0.80mol/L
��������
����ʵ�鲽������жϲ���˳��������Һ���ܽ���Ʒ���������ƵĹ���������Һ�ܽ�̼��ƣ�ʣ�������������������Һ�ζ������㷴Ӧ�����ᣬͨ��̼��ƺ����ᷴӦ������ϵ���㺬������������������д��ط�Ӧ����ʽ������������Һԭ����������������
��1������ʵ�鲽������жϲ���˳��������Һ���ܽ���Ʒ���������ƵĹ���������Һ�ܽ�̼��ƣ�ʣ�������������������Һ�ζ������㷴Ӧ�����ᣬͨ��̼��ƺ����ᷴӦ������ϵ���㺬�����ⶨ���̵���ȷ����˳��Ϊ�٢ڢܢۻ�ڢ٢ܢۣ��ʴ�Ϊ���٢ڢܢۻ�ڢ٢ܢۣ�
��2�������з����ķ�ӦΪ̼��ƺ��������������Ȼ��ơ�������̼��ˮ����Ӧ�����ӷ���ʽΪ��CaCO3+2H+=Ca2++CO2��+H2O������������Һ�����ᷴӦ�����Ȼ��ƺ�ˮ����Ӧ�����ӷ���ʽΪ��H++OH=H2O���ʴ�Ϊ��![]() ��
��![]() ��
��
��3��ÿ��ȡ��25.00mLHCl��4����100mL��������NaOH�ζ�ʱ����ҪС��100mL������ѡ�������ƿ���Ϊ100mL���ʴ�Ϊ��100mL��
��4����������Һ���Ƶ�ԭ�������ã���������NaOH��Һʱ��Ҫ����Ҫ������������ƽ���ձ�������ƿ(100mL)����Ͳ��ҩ�ס�����������ͷ�ιܣ��ʴ�Ϊ������ƿ(100mL)����Ͳ��ҩ�ס�����������ͷ�ιܣ�
��m(NaOH)=40g/mol��1.60mol/L��0.1L= 6.4g�����Գ�ȡ6.4gNaOH���壬�ʴ�Ϊ�� 6.4��
�����ձ���ȡNaOH����ʱ������˳��Ϊ��a.������ƽ��㡢d.��ȡС�ձ���������f.��¼���������ݡ�e.��ȡС�ձ���NaOH��������f.��¼���������ݡ�b.������Ż�����У�
�ʴ�Ϊ��adfefbc��
������Ŵ�λ��ʱ����Ʒ������Ӧ���������������ȥ��������������������Ϊ6.4g-1.4g=5g������ʵ������Ϊ5g-1.4g=3.6g���ʴ�Ϊ��3.6g��
��a.����ʱ������NaOH������������λ�õߵ�����Ŵ�λ��ʱ����Ʒ������Ӧ���������������ȥ�����������������Һ������ƫ�٣�����Ũ��ƫС��
b.δϴ���ձ���������������û����ȫת�Ƶ������У�����Ũ��ƫС��
c.����ҡ�Ⱥ���Һ����ڿ̶��ߣ����¼�ˮ���̶��ߣ�ʹ��Ũ��ƫС���ʴ�Ϊ��ƫС��ƫС��c.ƫС��
����Һϡ�����У����ʱ��ֲ��䣬��c1V1=c2V2����50mL��1.60mol/L=100mL��c2��c2=0.80mol/L���ʴ�Ϊ��0.80mol/L��


 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и������ʣ����ܰ���ͼ(��������ʾ��Ӧһ�����)��ϵת������
ѡ���� | a | b | c |
A | Al2O3 | NaAlO2 | Al(OH)3 |
B | Al | Al(OH)3 | Al2O3 |
C | AlCl3 | Al(OH)3 | NaAlO2 |
D | MgCl2 | Mg(OH)2 | MgO |
A. AB. BC. CD. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijС���Ի�ͭ��(��Ҫ�ɷ�Ϊ CuFeS2��������SiO2������)Ϊԭ���Ʊ�ͭ������ƷCuAlO2��һ�ֹ������£�

��֪�ٹ���1������Һ�к����������У�Cu2+��Fe2+��Fe3+������1����Ҫ�ɷ���SiO2��S��
��Cu(OH)2+4NH3��H2O=[Cu(NH3)4]2++2OH-+4H2O��
(1)�Ӳ�Ʒ���ȡ�������������ȽǶȿ��ǣ��Լ�A��B����������__________(�����)
a | b | c | d | |
A | HNO3 | NaClO | H2O2 (�ữ) | H2O2 (�ữ) |
B | Cu(OH)2 | NaOH | ��ˮ | Cu(OH)2 CO3 |
(2)������Ļ�ѧʽΪNH4Al(SO4)2��12H2O����Ϊ�Ʊ�ͭ�Ļ�����Ʒ�ṩ��Դ���������Һ��NH4+��A13+��H+��OH-��SO42��Ũ�ȴ�С����Ϊ_______________��
(3)����3�õ�A1(OH)3��Cu(OH)2��д����������������ѧ��Ӧ�ķ���ʽ��_________________��
(4)��λʱ��������ȡ��Cu2+�İٷ���(��Ϊ������)����ҺŨ�ȡ��¶ȹ�ϵ����ͼ��ʾ��
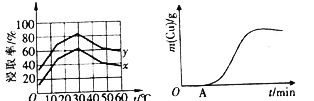
��20��ʱ��1L��Һ����Ч��ײ�ܴ�����x_________y(����>����<������=��)����ͬŨ���£��¶ȸ���30�棬�������������͵���Ҫԭ����_________________��
(5)��ͭ��ʯīΪ�缫�����������1��������Һ�Ʊ�ͭ��ͭ��������ͨ��ʱ���ϵ����ͼ��ʾ��д��OA�������ķ�Ӧ����ʽ��___________________________��
(6)�����£�Ksp[Fe(OH)3]=4.0��10-38�������Լ�B����pH=3ʱc(Fe3+)=_________mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ����A��ͨ���������ر�B��ʱ��C���ĺ첼������������������B����C ���ĺ첼������ɫ�������¼�����Һ����Dƿ��ʢ�ŵ���Һ������

��Ũ���� ��NaOH��Һ ��H2O �������Ȼ�����Һ
A.�٢�B.�٢�C.�ڢ�D.�ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������һ�ֵ��͵�ǿ��������
��1������ KMnO4 ������Һ�������� Cu2S ʱ�������ķ�Ӧ���£�8MnO4����5Cu2S��44H��=10Cu2����5SO2����8Mn2����22H2O
�ٻ�ԭ����Ϊ_____��
�ڱ�������Ԫ����_____
���������뻹ԭ�������ʵ���֮��Ϊ_____
��ÿ���� 2.24 L(�����)SO2��ת�Ƶ�����Ŀ��_____
��2���� KMnO4 ������Һ�������� CuS ʱ��Ҳ�ɽ� CuS ��Ӧ�� Cu2���� SO2��д�������ӷ�Ӧ����ʽ_____
��3��15.8g KMnO4�����ȷֽ��ʣ����� 15.0 g����ʣ�������������Ũ�����ڼ��������³�ַ�Ӧ�����ɵ������� A����������Ԫ���� Mn2+���ڣ������� A �����ʵ���Ϊ_____mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ǹ�ˮ����[Cr(CH3COO)2]2��2H2O(Ħ������376g/mol)��һ���������ռ�������ɫ���壬�ױ����������������ᣬ�����Ҵ���������ˮ�����ѡ����Ʊ�װ��(��ʡ�Լ��ȼ�֧��װ��)�Ͳ�������:
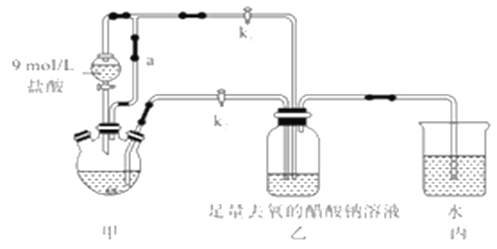
�ټ��װ��������,��������ƿ�����μ������п�ۣ�200mL0.200mol/LCrC13��Һ��
�ڹر�k2��k1,������Һ©�������������ƺõ��١�
�۴�������ƿ�ȵ���Һ������ɫ(Cr3+)��Ϊ����ɫ(Cr2+)ʱ������Һת�Ƶ�װ�����С������ִ�������ɫ����ʱ���رշ�Һ©����������
�ܽ�װ�����л������ٹ��ˡ�ϴ�Ӻ�������õ�11.28g[Cr(CH3COO)2]2��2H2O
��1��װ�ü�����ͨ��a��������________����μ����װ�õ��������Ƿ�����?__________��
��2�����ɺ���ɫ��������ӷ�Ӧ����ʽ____________________________��
��3�����������Һ�Զ�ת����װ�����е�ʵ�����Ϊ________________��
��4��װ�ñ���������________________��
��5��Ϊ�õ���������IJ�Ʒ��ϴ��ʱ��ʹ�õ��Ⱥ�˳��ѡ������ϴ�Ӽ�__________(�����)��
������ ������ˮ(�����ȴ) ����ˮ�Ҵ� ������
��6����ʵ����[Cr(CH3COO)2]2��2H2O�IJ�����______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ȼ��֬�ṹ�ĺ���Ϊ����[HOCH2CH(OH)CH2OH]����һ�����õķ���Ȼ��֬����ṹ�ĺ�����Ϊ����(C12H22O11)���÷���Ȼ��֬����ֱ���͵IJ���������(C17H33COOH)�����Ƿ�Ӧ���ã��䷴Ӧʾ��ͼ����ͼ��ʾ��ע�⣺ͼ�еķ�Ӧʽ������������˵����ȷ����

A. ������������֬������B. ����Ȼ��֬Ϊ�߷��ӻ�����
C. �÷���Ȼ��֬��ϡ���Ṳ�ȣ���ˮ������ڳ����¾�Ϊ��̬D. ����������ϡ�����������ˮ�⣬���տ����������л�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ����480 mL 0.5 mol��L-1Na2CO3��Һ����ʵ���ҽ������ơ�
I.��1����ͬѧ��������ƽӦ����________g Na2CO3��ĩ��ʹ������ƿǰ������е�һ��������______��
��2����ͼ�Ǹ�ͬѧ���Ƶ�һЩ�ؼ�����Ͳ���ͼ��

���ƹ��̵��Ⱥ�˳��Ϊ(����ĸA��F��д)________________��
��.������1mol/L��ϡ������Һ500mL
��1������Ҫ��������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ��������Ϊ___________mL(����������һλС��)�����ʵ������10mL ��25mL��50mL��Ͳ��Ӧѡ��_____________mL������Ͳ��á�
��2����������������ϡ������ҺŨ��ƫ�ߵ�����_____��
A���ܽ����Һû����ȴ�����¾�ת��
B��ת��ʱû��ϴ���ձ���������
C��������ƿ��ˮ����ʱ�۾�����Һ��
D������Ͳ��ȡŨ�����ϴ����Ͳ����ϴ��Һת�Ƶ�����ƿ
E��ҡ�Ⱥ���Һ����ڿ̶��ߣ��ּ�����ˮ���̶��ߣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com