
��10�֣����ɶ�����4��Ԫ�أ������⣩��ɵ�����X 4.83g�ܽ���1Lˮ�С��ü�ζ�������Һ�ĵȷ�������V��10.0mL��������0.028mol/L NaOH��Һ23.8mL������һ��ʱ�䣬���´���Һ��ȡ�ȷ�������V��10.0mL�����ζ�����0.028mol/L NaOH��Һ47.5mL�����ڶ��εζ�֮�����õ���Һ��Ϊ���ȷݣ���һ����Һ�м���MgSO4��Һ�����ɳ���5.2mg����ڶ�����Һ�м���BaCl2��Һ��������������Ϊ53.5mg��
1��ȷ��X�Ļ�ѧʽ��
2����дX��ˮ��Ӧ�Ļ�ѧ����ʽ��
3����pH��V��X��V��NaOH��Ϊ���껭���ڶ����ȷ�������pH�ζ���ͼ��
 ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д� 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
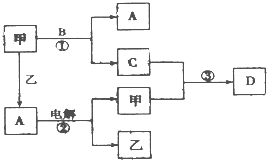 A��B��C��D���ɶ�����Ԫ����ɵ����ֳ����Ļ����DΪ����ɫ���壬�ס��������ֵ��ʣ���Щ���ʺͻ�����֮�������ͼ��ʾ��ת����ϵ��
A��B��C��D���ɶ�����Ԫ����ɵ����ֳ����Ļ����DΪ����ɫ���壬�ס��������ֵ��ʣ���Щ���ʺͻ�����֮�������ͼ��ʾ��ת����ϵ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
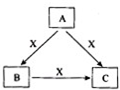 A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش�
A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
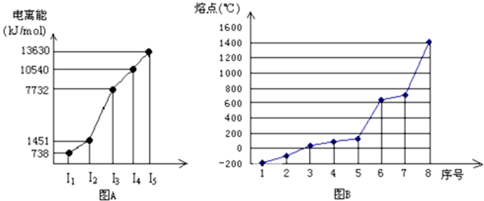
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com