
��ͭ����п���õ������ӣ����β���ֱ�ʢ�У���������Һ��������ͭ��Һ������������Һ�������ձ��У���ʱ��
(1)ͭƬ�Ϸ�������Ҫ��Ӧ�ĵ缫��Ӧʽ�ǣ���________________________��
��______________________________________________________________��
��_______________________________________________________________��
(2)����Ʒ�ھ���������ĵ����绯 ѧ��ʴ���أ�д���˵绯ѧ��ʴ�ĵ缫��Ӧʽ��
ѧ��ʴ���أ�д���˵绯ѧ��ʴ�ĵ缫��Ӧʽ��
����____________________________________________________________��
����____________________________________________________________��
(3)������ȥ������Ĥ��þƬ����Ƭ�ô��������ĵ������ӣ�����ʢ���ռ���Һ���ձ��У���ʱ���ֵ�����ָ��ƫת���жϴ�ԭ��ص�����������д���缫��Ӧʽ���ܷ�Ӧ����ʽ��
������________���缫��Ӧʽ��_____________________________________��������________���缫��Ӧʽ��_____________________________________��
�ܷ�Ӧ��________________________________________��
 ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д� �ݾ�ѵ������ϵ�д�
�ݾ�ѵ������ϵ�д� С����ȫ�ܼ��ϵ�д�
С����ȫ�ܼ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧ��̽��Na2O2��ˮ�ķ�Ӧ���ɹ�ʹ�õ��Լ��У�Na2O2������ˮ��KMnO4������Һ��MnO2����ͬѧȡһ����Na2O2��Ʒ�����ˮ��Ӧ������ȫ��Ӧ�õ���ҺX��һ����O2����ͬѧ�ƲⷴӦ�п���������H2O2��������ʵ��̽����
��1��д��Na2O2��ˮ��Ӧ�Ļ�ѧ����ʽ
��2�������ʵ��֤����ҺX�к���������
��3��ͨ������ʵ��֤����Һ��ȷʵ����H2O2��ȡ����X���Թ��У��μ�FeCl2��Һ���������ɺ��ɫ��������ƽ���з�Ӧ�����ӷ���ʽ��
H2O2 + Fe2+ + ==== Fe(OH)3¯��
��4����֪��ҺX��ʹ����KMnO4��Һ��ɫ��ͬʱ�ų���������ʱH2O2������ �ԣ����������ԭ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�жϼ���ƿ���Ƿ�װ�����������в�������ȷ���ǡ���
A. ��ƿ����һ���Ƿ��д̼�����ζ B. ��ʪ��ĺ�ɫʯ����ֽ����ƿ��
C. ��պ��Ũ����IJ���������ƿ�� D. ��պ��Ũ����IJ��������뼯��ƿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
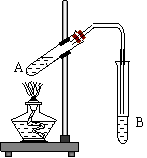
ijͬѧ��ʵ��������ͼ��ʾʵ��װ����ȡ�����������ش��������⣺
��1����A�Թ������ƺ������Ϊ3�U2���Ҵ������ᣬ���������ڣ���
��Ҳδ�й���ζҺ�����ɣ�ԭ���� ��
��2��B�Թ�����װ��ҺӦΪ �������������ɺ��ڸ���Һ
�� (��ϡ����¡�) �㣬�������Ʒ����IJ�������
�� ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�����ڳ��ʱ�����أ��ŵ�ʱ��ԭ��ء�Ǧ��������������������һ���������Ա��С���������һ���������Ա��С����������ڱ��С������Ľ�����������˵������ȷ����(����)
A�����ʱ���������ŵ�ʱ������
B�����ʱ������ԭ��Ӧ���ŵ�ʱ����������Ӧ
C�����ʱ���������ŵ�ʱ������
D����硢�ŵ�ʱ������������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
LiAl/FeS�����һ�����ڿ����ij��ص�أ��õ���������ĵ缫��ӦʽΪ��2Li����FeS��2e��===Li2S��Fe���йظõ�ص�����˵������ȷ����(����)��
A��LiAl�ڵ������Ϊ�������ϣ��ò�����Li�Ļ��ϼ�Ϊ��1��
B���õ�صĵ�ط�ӦʽΪ��2Li��FeS===Li2S��Fe
C�������ĵ缫��ӦʽΪ��Al��3e��===Al3��
D�����ʱ�����������ĵ缫��ӦʽΪ��Li2S��Fe��2e��===2Li����FeS
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Խ�����Ʒ���п���ʴ���������ӳ���ʹ��������
(1)����Ϊ���ı��洦����һ�ַ�����
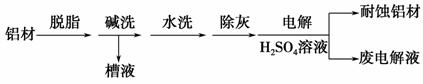
�ټ�ϴ��Ŀ���dz�ȥ���ı������Ȼ����Ĥ����ϴʱ��������ð����ԭ����_________________________________
(�����ӷ���ʽ��ʾ)��Ϊ����ϴ��Һ�е����Գ�����ʽ���գ�������Һ�м��������Լ��е�________��
a��NH3 b��CO2 c��NaOH d��HNO3
��������Ϊ��������H2SO4��Һ�е�⣬���ı����γ�����Ĥ�������缫��ӦʽΪ_______________________________________________________��
ȡ�����ϵ��Һ������NaHCO3��Һ��������ݺͰ�ɫ����������������ԭ����_________________________________________________________��
(2)��ͭ�ɷ�ֹ����Ʒ��ʴ�����ʱ��ͭ������ʯī��������ԭ����_________________________________________________________________��
(3)������ͼװ�ã�����ģ�����ĵ绯ѧ������
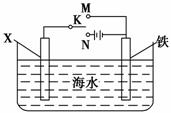
��XΪ̼����Ϊ�������ĸ�ʴ������KӦ����________������XΪп������K����M�����õ绯ѧ��������Ϊ______________________________ _____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ϵ������¶ȿ�ʵ�֡�ѩ�� ˮ
ˮ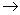 ˮ����
ˮ����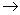 �������������ı仯���ڱ仯�ĸ��α��ƻ������Ӽ���Ҫ�������������( )
�������������ı仯���ڱ仯�ĸ��α��ƻ������Ӽ���Ҫ�������������( )
A.��������Ӽ����������Ǽ��Լ� B.�������������Լ�
C.��������Լ������Ӽ������� D.���Ӽ���������������Ǽ��Լ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��3���NO2��������ͨ���ٱ���NaHCO3��Һ����Ũ�����Na2 O2��(����ÿһ���ķ�Ӧ���dz�ֵ�)��������ˮ���ռ��������壬���ռ����������� (����)��
O2��(����ÿһ���ķ�Ӧ���dz�ֵ�)��������ˮ���ռ��������壬���ռ����������� (����)��
A��1���NO B��1���NO 2��
2��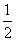 ���O2
���O2
C.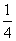 ���O2 D.
���O2 D.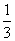
 ���NO
���NO
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com