

������������⣺
��1����ƿA�з�����Ӧ�Ļ�ѧ����ʽΪ��_________________����MnO2+4HCl��Ũ��![]() MnCl2+Cl2��+2H2O��
MnCl2+Cl2��+2H2O��
��2������ͨ������B��Ŀ����_______________________������ͨ������C��Ŀ����_____________________________��
��3��ʯӢ������D�з�����Ӧ�Ļ�ѧ����ʽ��__________________________________��
��4�����θ����E�м�ʯ�ҵ������ǣ�_________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
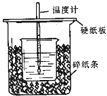 ��50mL 0.50mol/L������50mL 0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��50mL 0.50mol/L������50mL 0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
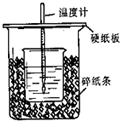 ��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ͭ������ϡ���ᣬ������������Һ����ͭ���������Σ��ֽ�һ������ͭƬ���뵽100mLϡ������������Ļ����Һ�У�ͭƬ��ȫ�ܽ⣨�������ε�ˮ�⼰��Һ����ı仯����
����ͭ������ϡ���ᣬ������������Һ����ͭ���������Σ��ֽ�һ������ͭƬ���뵽100mLϡ������������Ļ����Һ�У�ͭƬ��ȫ�ܽ⣨�������ε�ˮ�⼰��Һ����ı仯����2- 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
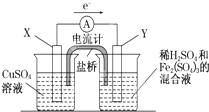
 ����ͼ��ʾ��װ���У���NaOH��Һ����м��ϡH2SO4�Ʊ�Fe��OH��2��ɫ������
����ͼ��ʾ��װ���У���NaOH��Һ����м��ϡH2SO4�Ʊ�Fe��OH��2��ɫ�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
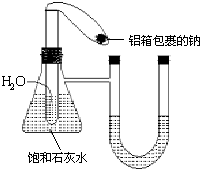 ����ͼ��ʾ��װ���У���ƿ��װ�б���ʯ��ˮ��С�Թ���װ��һ������ˮ����������һС�����������ŵ��ƣ�����װ�����������ã�δ��ӦǰU�ι�����Һ����ƽ���ֽ������еĽ����Ʒ���С�Թ�����ˮ��Ӧ��
����ͼ��ʾ��װ���У���ƿ��װ�б���ʯ��ˮ��С�Թ���װ��һ������ˮ����������һС�����������ŵ��ƣ�����װ�����������ã�δ��ӦǰU�ι�����Һ����ƽ���ֽ������еĽ����Ʒ���С�Թ�����ˮ��Ӧ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com