
| A���ƾ������Ȼ�̼ | B�����Ȼ�̼���� |
| C�����͡����� | D�����͡����� |
 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д� Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
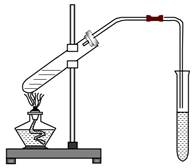









 ��1���ڴ��Թ�������һ���������Ҵ��������ŨH2SO4���Һ�ķ���Ϊ��
��1���ڴ��Թ�������һ���������Ҵ��������ŨH2SO4���Һ�ķ���Ϊ��  ��2��װ����ͨ�����ĵ���Ҫ���ڱ���Na2CO3��Һ��Һ�����ϣ����ܲ�����Һ
��2��װ����ͨ�����ĵ���Ҫ���ڱ���Na2CO3��Һ��Һ�����ϣ����ܲ�����Һ �У�Ŀ���ǣ�
�У�Ŀ���ǣ�  ��3��ŨH2SO4�����ã�
��3��ŨH2SO4�����ã�  ��4������Na2CO3�����ã�
��4������Na2CO3�����ã�  ��5���Թ��м����ʯ�����ã�
��5���Թ��м����ʯ�����ã�  ��6��ʵ�������ɵ������������ܶȱ�ˮ �����С������
��6��ʵ�������ɵ������������ܶȱ�ˮ �����С������ �� ����ζ��
�� ����ζ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
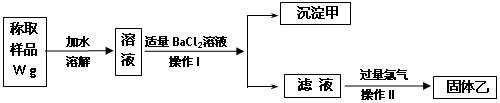
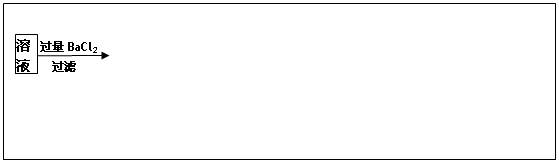
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
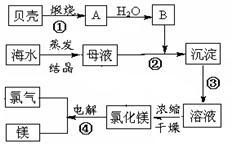
�鿴�𰸺ͽ���>>
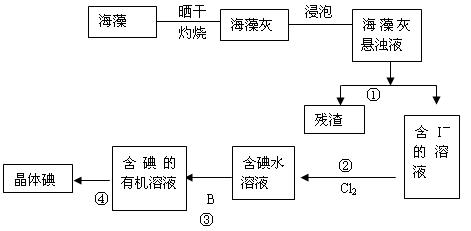
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��ʵ������ȡ��Ȳ�ķ�ӦΪ |
| B��Ϊ�˼ӿ췴Ӧ���ʿ��ñ���ʳ��ˮ����ˮ |
| C����ȼ��Ȳǰ�����鴿 |
| D���ø������������Һ����Ȳ�е����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢ� | B���ڢܢ� | C���٢ڢܢ� | D���٢ۢݢ�? |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
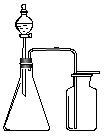
| A������ʯ�Һ��Ȼ�識��ȷ�Ӧ��NH3 |
| B��ͭ��ϡ���ᷴӦ��NO |
| C������������������̻����O2 |
| D���ý���п��ϡ���ᷴӦ��H2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ | B������ | C������ | D��һ������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com