
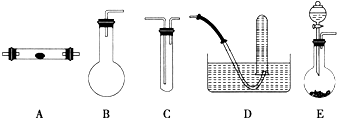
 ij��ѧ�о�С��ͨ�������������Ϸ���N2����ȡ���������ַ�����
ij��ѧ�о�С��ͨ�������������Ϸ���N2����ȡ���������ַ�����
| ||
| ||



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ||
| ||

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

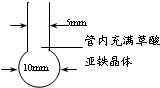 ������õ��˸ߴ��ȵ��������ۣ�
������õ��˸ߴ��ȵ��������ۣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
���ڴ����Na2SO3�ᱻ�����е���������������ij��ѧ��ȤС��ͨ��ʵ��ⲻ����ij��ˮNa2SO3�������ij̶ȡ�

��ش�
��1���������߿��ڵķ�Һ©�����ɳ���©������Ӧ��μ�����߿���װ�õ������ԣ�
��
��2��д��Bװ���з�Ӧ�����ӷ���ʽ ��
��3������a g Na2SO3��Ʒ������ƿ�У���Bװ�÷�Ӧ�����Һ�м�������BaC12��Һ��ַ�Ӧ�����ˡ�ϴ�ӡ���������ð�ɫ����b g������Ʒ�е�Na2SO3����������Ϊ ��
��4�������о����֣�����ʵ�鷽������ȱ�ݣ���ʹ��õ�Na2SO3��������ƫС���Է������е�ԭ���г�һ�����ɣ� ��
������ͬѧ�������һ��ʵ��װ������ͼ��

��5��ʵ���д���ƿ�в��ٲ����������P�ӵ�����˻�������һ�����Ŀ�������������Ŀ���� ��
��6�����ѳ�����a g Na2SO4��Ʒ�⣬ʵ���л�Ӧ�ⶨ�������� ����ͼ����ĸ��װ��ʵ��ǰ��������
�������������Լ�������ˮ�����ᡢϡ���ᡢBaC12��Һ��Ba��NO3��2��Һ��
��7����ͬѧ������ѡ������Լ������ⶨ��֪����Ϊa g��Na2SO3��Ʒ��Na2SO3����������������ʵ�鷽�������Ͽ��е��� ��
A������Ʒ�ܽ⣬���������ᣬ�ټ�����BaC12��Һ�����ˡ�ϴ�ӡ������������m1g
B������Ʒ�ܽ⣬���������ᣬ�ټ�����BaC12��Һ�����ˡ�ϴ�ӡ������������m2g
C������Ʒ�ܽ⣬�ӹ���BaC12��Һ�����˺���Һ���ɣ������ù���m3g
D������Ʒ��Һ���ӹ���Ba��NO3��2��Һ�����ˡ�ϴ�ӣ��ڳ����м����������ᣬ�ٹ��ˡ�ϴ�ӡ���������ù���m4g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��14�֣�I ѡ������ʵ�鷽���������ʣ������뷽�����������Ϊ
�ٷ���ˮ�����Ȼ�̼�Ļ����ڷ����Ȼ�����Һ��ɳ�ӵĻ���
�۴�����غ��Ȼ��ƵĻ����Һ�л������أ�
�� ����ƾ����е�Ϊ78.1�棩�ͼױ�(�е�Ϊ110.6��)�Ļ���
�ݷ���ʳ�κ͵�Ļ��� �� �ӵ�ˮ����ȡ�ⵥ�ʡ�
A������ B���ᾧ C����Һ D������ E����ȡ��Һ F������
II �����ڴ����Na2SO3�ᱻ�����е���������������ij��ѧ��ȤС��ͨ��ʵ��ⶨij��ˮNa2SO3�������ij̶ȡ�
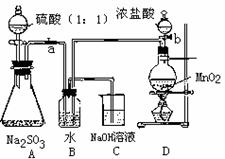
��ش𣺣�1��д��Bװ���з�Ӧ�����ӷ���ʽ
��2������a g Na2SO3��Ʒ������ƿ�У���Bװ�÷�Ӧ�����Һ�м�������BaC12��Һ��ַ�Ӧ�����ˡ�ϴ�ӡ���������ð�ɫ����b g������Ʒ�е�Na2SO3����������Ϊ ��
��3�������о����֣�����ʵ�鷽������ȱ�ݣ���ʹ��õ�Na2SO3��������ƫС���Է������е�ԭ���г�һ�����ɣ� ��
������ͬѧ�������һ��ʵ��װ������ͼ��
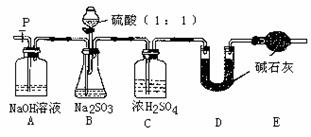
��4��ʵ���д���ƿ�в��ٲ����������P�ӵ�����˻�������һ�����Ŀ�������������Ŀ���� ��
��5�����ѳ�����a g Na2SO3��Ʒ�⣬ʵ���л�Ӧ�ⶨ�������� ����ͼ����ĸ��װ��ʵ��ǰ��������
�������������Լ�������ˮ�����ᡢϡ���ᡢBaC12��Һ��Ba��NO3��2��Һ��
��6����ͬѧ������ѡ������Լ������ⶨ��֪����Ϊa g��Na2SO3��Ʒ��Na2SO3����������������ʵ�鷽�������Ͽ��е��� ��
A������Ʒ�ܽ⣬���������ᣬ�ټ�����BaC12��Һ�����ˡ�ϴ�ӡ������������m1g
B������Ʒ�ܽ⣬���������ᣬ�ټ�����Ba��NO3��2��Һ�����ˡ�ϴ�ӡ������������m2g
C������Ʒ�ܽ⣬�ӹ���BaC12��Һ�����˺���Һ���ɣ������ù���m3g
D������Ʒ��Һ���ӹ���Ba��NO3��2��Һ�����ˡ�ϴ�ӣ��ڳ����м����������ᣬ�ٹ��ˡ�ϴ�ӡ���������ù���m4g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
(14��)���ڴ�ŵ�Na2SO3���ᱻ�����е���������������ij��ѧ��ȤС��ͨ��ʵ��ⶨij��ˮNa2SO3���������ij̶ȡ�
(��)��ͬѧ�������ͼʵ�顣

��ش�
(1)д��Bװ���з�Ӧ�����ӷ���ʽ ��
(2)д��Dװ���з�Ӧ�Ļ�ѧ����ʽ ��
(3)����ʼʵ��ʱ������������ƿ��Na2SO3��Ʒ����Ϊa g��ʵ�����Bװ�õ���Һ�м�������BaCl2��Һ��ַ�Ӧ�����ˡ�ϴ�ӡ���������ð�ɫ����b g������Ʒ��Na2SO3����������Ϊ ��
(4)�����о����֣�����ʵ�鷽������ȱ�ݣ���ʹ��õ�Na2SO3����������ƫС���Է������е�ԭ��(�г�һ������) ��
(��)��ͬѧ�������һ��ʵ��װ������ͼ��
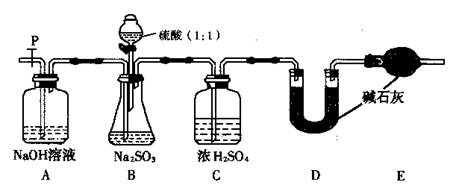
(5)ʵ���д���ƿ�в��ٲ����������P�ӵ�����˻�������һ�����Ŀ�������������Ŀ���� ��
(6)���ѳ�����a g Na2SO3����Ʒ�⣬ʵ���л�Ӧ�ⶨ�������� ��(��ͼ����ĸ)װ��ʵ��ǰ��������
(��)���������Լ�������ˮ�����ᡢϡ���ᡢBaCl2��Һ��Ba(NO3)2��Һ��
(7)��ͬѧ������ѡ������Լ������ⶨ��֪����Ϊag��Na2SO3����Ʒ��Na2SO3����������������ʵ�鷽�������Ͽ��е��� ��
A. ����Ʒ�ܽ⣬���������ᣬ�ټ�����BaCl2��Һ�����ˡ�ϴ�ӡ���������ó���m
B������Ʒ�ܽ⣬���������ᣬ�ټ�����BaCl2��Һ�����ˡ�ϴ�ӡ���������ó���m2 g
C������Ʒ�ܽ⣬�ӹ���BaCl2��Һ�����˺���Һ���ɣ������ù���m3 g
D������Ʒ�ܽ⣬�ӹ���Ba(NO3)2��Һ�����ˡ�ϴ�ӣ��ڳ����м����������ᣬ�ٹ��ˡ�ϴ�ӡ���������ù���m4 g
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com