


 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1.75a% | B��1.25a% | C��1��1.75a% | D��1��1.25a% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A�������£�0.1mol̼���ƾ����к���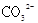 �ĸ���Ϊ0.1NA �ĸ���Ϊ0.1NA |
| B����״���£�11.2L������C��H����ĿΪ3NA |
| C�����³�ѹ�£�54 g��ˮ��D2O����ˮ������Ϊ3NA |
| D����NA��Cl2����ͨ��������ˮ�У���ת�Ƶĵ�������ΪNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����³�ѹ�£�22.4 L CH4�к���4 nA��C-H�� |
| B��1 mol Fe������ϡ���ᷴӦ��ת��2 nA������ |
| C��0.5 mol��L��1 FeCl3��Һ�к���1.5 nA��Cl�� |
| D�������£�22g CO2����nA����ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���أ�35a/(22.4V��) |
| B��c ��a/(22.4V)mol/L |
| C��������ˮ���ټ���������Ũ�ȵ�ϡ�������Һǡ�ó����� |
| D��������ˮ���ټ���V mLˮ��������Һ����������С��0.5�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1mol��L-1 | B��2.5 mol��L-1 | C��5 mol��L-1 | D��2 mol��L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��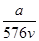 mol��L��1 mol��L��1 | B��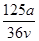 mol��L��1 mol��L��1 |
C��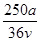 mol��L��1 mol��L��1 | D��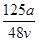 mol��L��1 mol��L��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����״���£�22��4L����ȵ������������Ļ�������еķ���������Ϊ2NA |
| B����״���£�22��4Lˮ�к���NA��O��H�� |
| C������ͭ��1L18mol��L-1Ũ���ᷴӦ��������SO2��������Ϊ9NA |
| D��100mL0��1mol��L-1HNO3��Һ�к���0��01NA��NO3�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com