
��10�֣�
ij��ɫ����Һ�п��ܴ�������Ag����Mg2����Fe3���еļ������ӡ�
(1)�����κ�ʵ��Ϳ��Կ϶�ԭ��Һ�в����ڵ������� ��
(2)ȡ����ԭ��Һ�������ϡ���ᣬ�а�ɫ�������ɣ��ټ������ϡ���ᣬ��ɫ��������ʧ��˵��ԭ��Һ�п϶��е����� �� �йص����ӷ�ӦʽΪ ��
(3)ȡ(2)����Һ�������NaOH��Һ�����ְ�ɫ������˵��ԭ��Һ�п϶����ڵ������� ��
(4)ԭ��Һ�п��ܴ������ڵ�������������A��D�еģ�����ţ� ��
A��Cl�� B��NO3�� C��CO32�� D��OH��
 ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.![]() ��Cl-��
��Cl-��![]() ��K+ B.Na+��K+��Cl-��
��K+ B.Na+��K+��Cl-��![]()
C. ![]() ��K+��Cl-��Na+ D.
��K+��Cl-��Na+ D. ![]() ��Mg2+��Cl-��Al3+
��Mg2+��Cl-��Al3+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��4�֣�ij��ɫ����Һ��Ͷ��������ɷų�H2�������������ӣ�Mg2+��Cu2+��Ba2+��H+��Ag+��SO42-��HCO3����OH�������ж������������ʱ��������Щ���ӿɴ����ڴ���Һ�У�
��1��������Al3+ʱ���ɴ��ڵ������� ����2�֣�
��2��������AlO2��ʱ���ɴ��ڵ������� ����2�֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ��ԭ�ر�����ѧ��һ�������¿���ѧ�Ծ����������� ���ͣ������
��6�֣�ij��ɫ����Һ��Ͷ��������ɷų�H2�������������ӣ�Mg2+��Cu2+��H+��Cl-��K+��OH����CO32��,���ж�������������£������Ӵ����ڴ���Һ�е������
��1����һ�����������Һ�еμ���ɫʯ���ʺ�ɫ�������Һ��һ���� ���ӣ�һ��û�� ���ӣ������� ���ӣ�
��2����һ�����������Һ�еμ���ɫʯ������ɫ�������Һ��һ���� ���ӣ�һ��û�� ���ӣ������� ���ӡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ɫ����Һ�п��ܴ���Na+��Ag+��Ba2+��Fe3+��AlO2����CO32����SO32����SO42����ȡ����Һ�������ʵ�飬ʵ������¼���£�
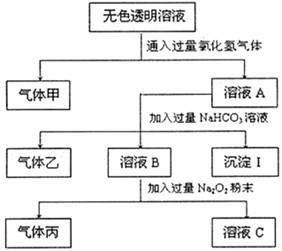
��1���������κλ�ѧ��Ӧ�����жϸ���Һ�п϶�û�е������� ���������ͬԪ�صĵ������������ȼ�յ������� �����������и����ӵ��Ȼ���ı�����Һ������ڵ�ˮ�����ɺ��ɫҺ�壬��Ӧ�Ļ�ѧ����ʽ�� ��
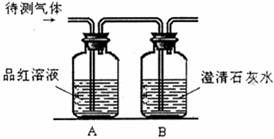
��2��Ϊȷ������ijɷ֣�����ͼ��ʾװ��ʵ�飬��Aƿ��Һ��ɫ��ɫBƿ��Һ���а�ɫ�������ɣ�����������ɿɱ�ʾΪ ��
��3������ɫ����Һ��һ�����ڵ������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com