
���ڱ�ǰ�����ڵ�Ԫ��a��b��c��d��e��ԭ��������������a�ĺ����������������������ͬ��b�ļ۵��Ӳ���δ�ɶԵ�����3����c������������Ϊ���ڲ��������3����d��cͬ�壻e�������ֻ��1�����ӣ����������18�����ӡ��ش��������⣻
(1)b��c��d�е�һ������������________(��Ԫ�ط���)��e�ļ۲���ӹ��ʾ��ͼΪ________��
(2)a������Ԫ���γɵĶ�Ԫ���ۻ������У����ӳ������Σ��÷��ӵ�����ԭ�ӵ��ӻ���ʽΪ______�������мȺ��м��Թ��ۼ����ֺ��зǼ��Թ��ۼ��Ļ�������________(�ѧʽ��д������)��
(3)��ЩԪ���γɵĺ������У����ӵ�����ԭ�ӵļ۲���Ӷ���Ϊ3������________������������ṹ������________��(�ѧʽ)
(4)e��c�γɵ�һ�����ӻ�����ľ���ṹ��ͼ(a)����e���ӵĵ��Ϊ________��
(5)��5��Ԫ���γɵ�һ��1��1�����ӻ������У������ӳ�������ṹ�������ӳ����������İ�����ṹ[��ͼ(b)��ʾ]��
 ������
������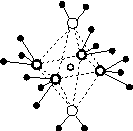
(a)����������������������(b)
�û������У�������Ϊ________���������д��ڵĻ�ѧ��������________���û��������ʱ����ʧȥ�������________���ж�������______________________________________________��
(1)N��
(2)sp3��H2O2��N2H4
(3)HNO2��HNO3��H2SO3
(4)��1
(5)SO �����ۼ�����λ����H2O
�����ۼ�����λ����H2O
H2O��Cu2���������NH3��Cu2������
[����] ��ԭ��������С�Һ����������������Ӳ�����ͬ��ȷ��aΪ��Ԫ�أ��ɼ۵��Ӳ��е�δ�ɶԵ�����3��ȷ��bΪ��Ԫ�أ�������������Ϊ���ڲ��������3��ȷ��cΪ��Ԫ�أ���d��cͬ����ȷ��dΪ��Ԫ�أ���e�������ֻ��1�������Ҵ������18������ȷ��eΪͭԪ�ء�(1)��һ������һ����ɣ�ͬ���ڴ������ҵ�����ͬ����������µݼ�������ԭ�ӹ����ȫ����������ȫ��״̬ʱ�ȶ�����һ�����ܷ����Ĵ���ͭ�ļ۵����Ų��ɻ����۵��ӹ��ʾ��ͼ��(2)�����ҳ������εķ�����NH3��Nԭ���ӻ���ʽΪsp3��O��N��C���⻯������к��зǼ��Թ��ۼ��Ļ�������H2O2��N2H4��C2H6��C6H6�ȡ�(4)Cu��O���γ����ֻ�������ݾ�̯������ԭ������ȷ��������Ļ�ѧʽΪCu2O����Cu����(5)5��Ԫ���γɵ�1��1�����ӻ������У������ӳ�������ṹ��ΪSO ����ͼ(b)�е������ӽṹ��֪����Cu2����4��NH3��2��H2O�������ӷ���Ϊ[Cu(NH3)4(H2O)2]2�������й��ۼ�����λ�������Ȼ�����ʱ������λ��ǿ��ȷ������ʧȥ�ijɷ֡�
����ͼ(b)�е������ӽṹ��֪����Cu2����4��NH3��2��H2O�������ӷ���Ϊ[Cu(NH3)4(H2O)2]2�������й��ۼ�����λ�������Ȼ�����ʱ������λ��ǿ��ȷ������ʧȥ�ijɷ֡�


 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ij�л�����ӵı���ģ�ͣ��йظ����ʵ��ƶϲ���ȷ���ǣ� ��

A�������п��ܺ����ǻ� B�������п��ܺ����Ȼ�
C�������п��ܺ��а��� D�������ʵķ���ʽ����ΪC3H6O3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ؾ��ں��зḻ��������ʳ����Դ��ʳ�����ճ������еı���Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϡ�
(1)���ⶨ���������´����к�������K����Ca2����Mg2����Fe3�����������ӣ�ij�о���ѧϰС����ʵ�����ᴿNaCl���������£�
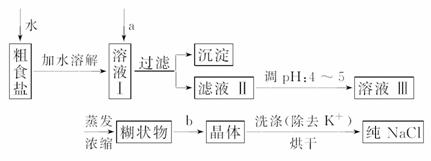
���ṩ���Լ�������Na2CO3��Һ������K2CO3��Һ��NaOH��Һ��BaCl2��Һ��Ba(NO3)2��Һ��75%�Ҵ���Һ��CCl4����������Ʒ��ѡ��
������ȥ��Һ�е�Ca2����Mg2����Fe3����SO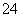 ��ѡ��a���������������Լ������μ�˳������Ϊ_________________________________________________________(ֻ�ѧʽ)��
��ѡ��a���������������Լ������μ�˳������Ϊ_________________________________________________________(ֻ�ѧʽ)��
b��������������____________��
��ϴ�ӳ�ȥNaCl������渽��������KCl��Ӧѡ���Լ���____________����pH��ֽ�ⶨ��Һ��pHֵ�ķ�����______________________________________________��
(2)���ᴿ��NaCl����500 mL��2.5 mol��L��1��NaCl��Һ�������������ձ���������ƽ(���������)��ҩ�ף��������⣬����Ҫ____________________________________(����������)��Ӧ��ȡNaCl________ g��
(3)���в����ᵼ������NaCl��ҺŨ��ƫ�ߵ���___________________________��
A��������Ϻ�����ҡ�ȣ��ٽ�����ƿ����ʵ��̨�ϣ�����Һ����ڿ̶��ߣ�����������ˮ���̶���
B��δ��ϴ���ձ��ڱڵ���Һת������ƿ
C������ʱ�����ӿ̶���
D��ת����Һ֮ǰ������ƿ������������ˮ
E������ʱ����ƽָ��ָ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��25���ˮ��Һ�У�AgX��AgY��AgZ��������ˮ���������ܽ�ƽ�⡣���ﵽƽ��ʱ����Һ������Ũ�ȵij˻���һ������(�˳�����Ksp��ʾ��Ksp��ˮ��KW����)���磺
AgX(s) Ag����X����Ksp(AgX)��c(Ag��)��c(X��)��1.8��10��10
Ag����X����Ksp(AgX)��c(Ag��)��c(X��)��1.8��10��10
AgY(s)  Ag����Y����Ksp(AgY)��c(Ag��)��c(Y��)��1.0��10��12
Ag����Y����Ksp(AgY)��c(Ag��)��c(Y��)��1.0��10��12
AgZ(s)  Ag����Z����Ksp(AgZ)��c(Ag��)��c(Z��)��8.7��10��17
Ag����Z����Ksp(AgZ)��c(Ag��)��c(Z��)��8.7��10��17
����˵���������(����)
A�������������ڳ������ܽ��������AgZ
B����AgY�ܽ���ˮ�������м���AgX����c(Y��)��С
C����25��ʱ��ȡ0.188 g AgY(��Է�������Ϊ188)�������100 mLˮ��(������Һ����ı仯)������Һ��c(Y��)��1.0��10��4 mol/L
D�������ܽ�ƽ��Ľ������������ģ���������ı�ʱ��ƽ��Ҳ�ᷢ���ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Na��Cu��O��Si��S��Cl�dz���������Ԫ�ء�
(1)Naλ��Ԫ�����ڱ���________���ڵ�________�壻S�Ļ�̬ԭ�Ӻ�����________��δ�ɶԵ��ӣ�Si�Ļ�̬ԭ�Ӻ�������Ų�ʽΪ________________________��
(2)�á�>����<����գ�
| ��һ������ | ���Ӱ뾶 | �۵� | ���� |
| Si____S | O2��____Na�� | NaCl____Si | H2SO4____HClO4 |
(3)CuCl(s)��O2��Ӧ����CuCl2(s)��һ�ֺ�ɫ���塣��25 �桢101 kPa�£���֪�÷�Ӧÿ����1 mol CuCl(s)������44.4 kJ���÷�Ӧ���Ȼ�ѧ����ʽ��________________________________________________________________________
________________________________________________________________________��
(4)ClO2������ˮ�ľ�������ҵ�Ͽ���Cl2����NaClO2��Һ��ȡClO2��д���÷�Ӧ�����ӷ���ʽ�����������ת�Ƶķ������Ŀ��________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ش��������⣺
(1)31Ga��̬ԭ�ӵĺ�������Ų�ʽ��________________��ij�ְ뵼�������Ga��As����Ԫ����ɣ��ð뵼����ϵĻ�ѧʽ��________���侧��ṹ���Ϳ���Ϊ________��
(2)ά����B1����Ϊ��ø�����ǵĴ�л�����б�����ϵͳ�����á������ʵĽṹʽΪ

���¹���ά����B1��˵����ȷ����________��
A��ֻ���Ҽ��ͦм�
B�����й��ۼ��������Ӽ�
C�������ʵ��۵���ܸ���NaCl
D������������������
(3)ά����B1��������ˮ�Ĺ�����Ҫ�˷����������������________��
A�����Ӽ������ۼ�
B�����Ӽ�����������ۼ�
C����������»���
D�����Ӽ�����������»���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��.�����Ƶ�±����(NaX)���±����(SiX4)������������ȷ����(����)
A��SiX4��ˮ��
B��SiX4�ǹ��ۻ�����
C��NaX��ˮ��
D��NaX���۵�һ�����SiX4
��.̼Ԫ�صĵ����ж�����ʽ����ͼ������C60��ʯī�ͽ��ʯ�Ľṹͼ��

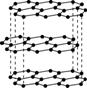

����������C60����������������ʯī�����������ʯ����
�ش��������⣺
(1)���ʯ��ʯī��C60��̼���ܵȶ���̼Ԫ�صĵ�����ʽ�����ǻ�Ϊ________________��
(2)���ʯ��ʯīϩ(ָ����ʯī)��̼ԭ�ӵ��ӻ���ʽ�ֱ�Ϊ________��________��
(3)C60����________���壬ʯī����________���塣
(4)ʯī�����У�����C��C���ļ���Ϊ142 pm�������ʯ��C��C���ļ���Ϊ154 pm����ԭ���ǽ��ʯ��ֻ����C��C���________���ۼ�����ʯī���ڵ�C��C�䲻������________���ۼ�������________����
(5)���ʯ��������________��̼ԭ�ӡ���̼ԭ�Ӱ뾶Ϊr�����ʯ�����ı߳�Ϊa������Ӳ��Ӵ�ģ�ͣ���r��____________a����ʽ��ʾ̼ԭ���ھ����еĿռ�ռ����____________________(��Ҫ�������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ӦA+3B=4C+2D���ڲ�ͬ�����·�Ӧ����ƽ����Ӧ����v(X)����ʾ��Ӧ����������ʻ���������������ʣ����£����з�Ӧ���������� ��
��A��v(A)=0.4mol/(L��s)������B��v(B)=0.8mol/(L��s)
C��v(C)=1.2mol/(L��s)������ D��v(D)=0.7mol/(L��s)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������й��ݡ�����ݵȽ�����ʹ�õĹ����أ��ܹ��ʴ�4���ߣ�������������֮������й�������ȷ����(����)
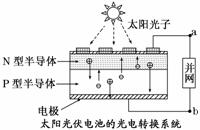
A���������ǽ�̫����ת��Ϊ����
B���������ǽ���ѧ��ת��Ϊ����
C��������a����b
D��ͼ��N�Ͱ뵼��Ϊ������P�Ͱ뵼��Ϊ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com