
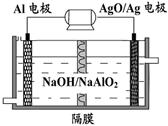
1��һ������﮵���ǽ���ѧʽΪLi4Ti5O12��������Ϊ��ص��������ϣ��ڷŵ�Ĺ����б�Ϊ��ѧʽΪLi4Ti5O12�����ʡ�
��Li4Ti5O12��TiԪ�صĻ��ϼ�Ϊ ��﮵�ص�ͻ���ŵ��� ��
�ڸ�﮵����һ�ֶ��ε�أ��ŵ�ʱ�ĸ�����ӦʽΪ �����ʱ��������ӦʽΪ ��
��2����������ԭ�ζ����ⶨ�Ʊ��õ���TiO2�����е�TiO2��������������һ�������£���TiO2�ܽⲢ��ԭΪTi3+������KSCN��Һ��Ϊָʾ������NH4Fe��SO4��2����Һ�ζ�Ti3+��ȫ������Ti4+��
��TiCl4ˮ������TiO2��xH2O�Ļ�ѧ����ʽΪ ��
�ڵζ��յ�������� ��
�۵ζ�����ʱ����ȡTiO2����0��2g������0.1mol��L-1 NH4Fe��SO4��2����Һ20ml����TiO2����������Ϊ____ ��
�����ڵζ��յ㣬��ȡ�ζ��̶ܿ�ʱ�����ӱ���Һ��Һ�棬ʹ��ⶨ��� ���ƫ����ƫС������Ӱ�족��o
��3����֪��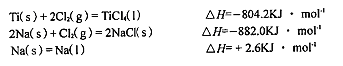
��TiCl4��I��+4Na��l��=Ti��s��+4NaCl��s���ġ�H= KJ��mol-1��
��1����+4(1��)���С���������ߡ�Я�����㡣��1�֣�
��Li-e-=Li+��1�֣�Li7Ti5O12-3e-=Li4Ti5O12+3Li+��2�֣�
��2����TiCl4+(x+2)H2O=TiO2?xH2O��+4HCl��2�֣�����Һ��ɺ�ɫ(1��)
��80%��2�֣���ƫС��1�֣�
��3��-970.2��2�֣�
������1���ٸ��ݻ����ﻯ�ϼ۴����͵����㣬�Ϊ+1�ۣ���Ϊ-2�ۣ�����Ϊ+4�ۣ��ڷŵ緢��ԭ��ط�Ӧ������Li-e-=Li+����緢�����ط�Ӧ����������ӵ�Դ����������ʧȥ���ӣ�����������Ӧ��Li7Ti5O12-3e-=Li4Ti5O12+3Li+��
��2����TiCl4ˮ������TiO2?xH2O������TiO2�Ļ�ѧʽ��Ӧ��2molˮ���뷴Ӧ���ʷ���ʽΪTiCl4+(x+2)H2O=TiO2?xH2O��+4HCl����n(NH4Fe��SO4��2)=0.1mol/L��0.02L=0.002mol,�˷�Ӧ��������ԭ��Ӧ�����ݵ��ӵ�ʧ�غ㣬n(NH4Fe��SO4��2)=n(TiO2),m(TiO2)=0.002mol��80g��mol-1,0.002��80/0.2=0.80���ܵζ��յ㣬���Ӷ�������������ֵƫ���������ֵƫС���ʲⶨ���ƫ�͡���3�����ݸ�˹���ɣ������еķ���ʽ��ϾͿɵõ������
TiCl4(l)=Ti(s)+2Cl2(g),��H=804.2KJ��mol-
4Na(s)+2Cl2(g)="4NaCl(s)" ��H=-2��882.0KJ��mol-
4Na(l)="4Na(s)" ��H=-4��2.6KJ��mol-
����������ӣ��͵õ���H=-970.2KJ��mol-


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ҹ��Ǹ��������,��������Ϊ�����һ,��¯��������Ϊ�ձ����������.
I. ��֪��2CO(g)+ O2(g)��2CO2(g),��H��-566 kJ��mol-1
2Fe(s)+ 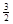 O2(g)��Fe2O3(s),��H��-825.5 kJ��mol-1
O2(g)��Fe2O3(s),��H��-825.5 kJ��mol-1 ��Ӧ��Fe2O3(s)+ 3CO(g)
��Ӧ��Fe2O3(s)+ 3CO(g) 2Fe(s)+ 3CO2(g),��H��______ kJ��mol-1.
2Fe(s)+ 3CO2(g),��H��______ kJ��mol-1.
��. ��Ӧ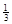 Fe2O3(s)+ CO(g)
Fe2O3(s)+ CO(g)
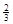 Fe(s)+ CO2(g)��1000���ƽ�ⳣ������4.��һ���ݻ�Ϊ10L���ܱ�������,1000��ʱ����Fe��Fe2O3��CO��CO2��1.0mol,��Ӧ����l0min��ﵽƽ��.
Fe(s)+ CO2(g)��1000���ƽ�ⳣ������4.��һ���ݻ�Ϊ10L���ܱ�������,1000��ʱ����Fe��Fe2O3��CO��CO2��1.0mol,��Ӧ����l0min��ﵽƽ��.
��1��CO��ƽ��ת����=____________.
��2�������CO��ƽ��ת����,�ٽ�Fe2O3��ת��,�ɲ�ȡ�Ĵ�ʩ��________.
a����߷�Ӧ�¶�
b������Ӧ��ϵ��ѹǿ
c��ѡȡ���ʵĴ���
d����ʱ���ջ��Ƴ�����CO2
e�������ʯ,ʹ����ƽ���������ֽӴ�
��.��¯���������ķ����е�CO�ɽ��л���,ʹ����һ�������º�H2��Ӧ�Ʊ��״�:
CO(g)+ 2H2(g) CH3OH(g).�����ͼʾ�ش���������:
CH3OH(g).�����ͼʾ�ش���������: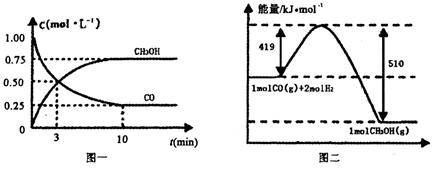
��1���ӷ�Ӧ��ʼ��ƽ��,��H2Ũ�ȱ仯��ʾƽ����Ӧ����v(H2)=________.
��2�������¶Ⱥ�������ͬ�������ܱ�������,����ͬ��ʽͶ�뷴Ӧ��,��÷�Ӧ�ﵽƽ�ⅼ���й��������±���
| ���� | ��Ӧ��Ͷ����� | ��Ӧ��� ת���� | CH3OH��Ũ�� | �����仯 (Q1��Q2��Q3������0) |
| �� | 1mol CO��2mol H2 | ��1 | c1 | �ų�Q1kJ���� |
| �� | 1mol CH3OH | ��2 | c2 | ����Q2kJ���� |
| �� | 2mol CO��4mol H2 | ��3 | c3 | �ų�Q3kJ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
2013������������������Ű�ҹ��ж������������У�����β����ȼúβ������ɿ�����Ⱦ��ԭ��֮һ��
��l������β����������Ҫԭ��Ϊ��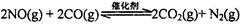 �����ܱ������з����÷�Ӧʱ��c��CO2�����¶ȣ�T���������ı������S����ʱ�䣨t���ı仯���ߣ���ͼ��ʾ���ݴ��жϣ�
�����ܱ������з����÷�Ӧʱ��c��CO2�����¶ȣ�T���������ı������S����ʱ�䣨t���ı仯���ߣ���ͼ��ʾ���ݴ��жϣ�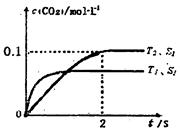
�ٸ÷�Ӧ�Ħ�H______0��ѡ���������<������
�ڸ÷�Ӧ��ƽ�ⳣ������ʽΪ____________________
����T2�¶��£�0 ~ 2s�ڵ�ƽ����Ӧ����v��N2��_______��
�ܵ��������������һ��ʱ�����������������ѧ��Ӧ���ʡ��������ı����S1��S2������ͼ�л���c��CO2����T1��S2�����´ﵽƽ������еı仯���ߡ�
�ݸ÷�Ӧ�ھ��ȡ����ݵ��ܱ���ϵ�н��У�����ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ�̴ﵽƽ��״̬����__________������ţ���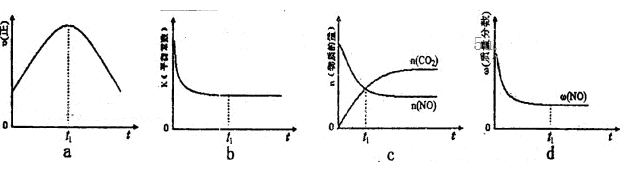
��2��ֱ���ŷ�úȼ�ղ������������������صĻ������⡣úȼ�ղ����������������������CH4����ԭNOx�������������������Ⱦ�����磺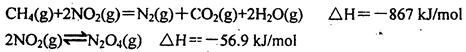
д��CH4����ԭN2O4(g)����N2(g)��CO2(g)��H2O(g)���Ȼ�ѧ����ʽ�ߣߣߣߣߣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ŀǰ����������������Ⱦ�ж��ַ�����
��1����CH4����ԭ��������������������������Ⱦ����֪��
��CH4(g)��4NO2(g)��4NO(g)��CO2(g)��2H2O(g) ��H����574 kJ��mol��1
��CH4(g)��4NO(g)��2N2(g)��CO2(g)��2H2O(g) ��H����1160 kJ��mol��1
��H2O(g)��H2O(l) ��H����44��0 kJ��mol��1
д��CH4 (g)��NO2 (g)��Ӧ����N2 (g) ,CO2(g)��H2O(l)���Ȼ�ѧ���� ʽ_____________________
��2���û���̿��ԭ��������������йط�ӦΪ��C(s)��2NO(g) N2(g)��CO2(g)ij�о�С��������ܱ���������һ�����Ļ���̿��NO�����£�T��C)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ������
N2(g)��CO2(g)ij�о�С��������ܱ���������һ�����Ļ���̿��NO�����£�T��C)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ������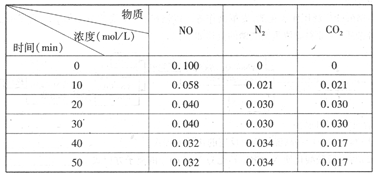
�ٲ�����Ϊ�жϷ�Ӧ�ﵽ��ѧƽ��״̬������ ��_______
A��������CO2��Ũ�ȱ��ֲ���
B��v����N2��="2" v����NO��
C��������ѹǿ���ֲ���
D�����������ܶȱ��ֲ���
E����������ƽ����Է����������ֲ���
����T��Cʱ���÷�Ӧ��ƽ�ⳣ��Ϊ_______(������λС��)��
����30 min,�ı�ijһ����,��Ӧ���´ﵽƽ��,��ı��������_______
��3����ѧ�������о����ô������������ٷɻ�β���е�NO��COת���CO2��N2,�䷴ӦΪ��
2CO��2NO N2��2CO2 ��H<0�о���������ʹ�õ���������ʱ����������ıȱ���������ѧ��Ӧ���ʣ�Ϊ�˷ֱ���֤�¶ȡ������ıȱ�����Ի�ѧ�� Ӧ���ʵ�Ӱ����ɡ�ijͬѧ���������ʵ�飬����ʵ�������Ѿ������±��С�
N2��2CO2 ��H<0�о���������ʹ�õ���������ʱ����������ıȱ���������ѧ��Ӧ���ʣ�Ϊ�˷ֱ���֤�¶ȡ������ıȱ�����Ի�ѧ�� Ӧ���ʵ�Ӱ����ɡ�ijͬѧ���������ʵ�飬����ʵ�������Ѿ������±��С�
���ϱ���:a=_______,b=________,e=________
�����ڸ���������ͼ�У������ϱ���ʵ��II��ʵ��III�����»��������NOŨ����ʱ��仯����������ͼ,��������Ӧ��ʵ����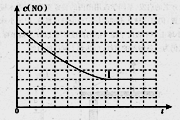
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ��Ӧ�仯���̼�������о�����Ҫ��ش����⣺
��1�����ڷ�Ӧ�����������仯���о���
��2CO��g��+O2��g��=2CO2��g����H= kJ��mol-1��
��2�����ڷ�Ӧ���ʺ��ȵ��о���
��ҵ�������ص�ԭ������NH3��CO2Ϊԭ�Ϻϳ�����[CO(NH2)2]����Ӧ�Ļ�ѧ����ʽΪ��
2NH3 (g)+ CO2 (g)  CO(NH2)2 (l) + H2O (l)���÷�Ӧ��ƽ�ⳣ����K�����¶ȣ�T / �棩��ϵ���£�
CO(NH2)2 (l) + H2O (l)���÷�Ӧ��ƽ�ⳣ����K�����¶ȣ�T / �棩��ϵ���£�
| T / �� | 165 | 175 | 185 | 195 |
| K | 111.9 | 74.1 | 50.6 | 34.8 |
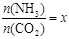 ����ͼ��1���ǰ�̼�ȣ�x����CO2ƽ��ת���ʣ������Ĺ�ϵ��������x����������ԭ���� ��
����ͼ��1���ǰ�̼�ȣ�x����CO2ƽ��ת���ʣ������Ĺ�ϵ��������x����������ԭ���� ��

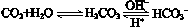 ��ʹ����ѪҺpH������7.35��7.45������ͻᷢ�����ж�����ж�����pH��c(HCO3-)��c(H2CO3)�仯��ϵ���±���
��ʹ����ѪҺpH������7.35��7.45������ͻᷢ�����ж�����ж�����pH��c(HCO3-)��c(H2CO3)�仯��ϵ���±���| c(HCO3-)��c(H2CO3) | 1.0 | 17.8 | 20.0 | 22.4 |
| pH | 6.10 | 7.35 | 7.40 | 7.45 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ǵ����Ϻ����ḻ��һ��Ԫ�أ���Ԫ�صĵ��ʺͻ������ڹ�ũҵ����������������Ҫ��;��
��1���������������仯ʾ��ͼ��
д��CO��NO2��Ӧ����NO��CO2���Ȼ�ѧ����ʽ
��2���ڹ̶�������ܱ������У��������»�ѧ��Ӧ��N2(g)+3H2(g)  2NH3 (g) ��H<0��
2NH3 (g) ��H<0��
��ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±������ж�K1 K2���>������=����<����
| T /K | 298 | 398 |
| ƽ�ⳣ��K | K1 | K2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
CO2��CH4��������Ҫ���������壬ͨ��CH4��CO2��Ӧ�������ֵ��ѧƷ��Ŀǰ���о�Ŀ�ꡣ
��1��250��ʱ�������Ͻ�Ϊ��������4 L������ͨ��6 mol CO2��6 mol CH4���������·�Ӧ��CO2(g)��CH4(g) 2CO(g)��2H2(g)��ƽ����ϵ�и��������������±���
2CO(g)��2H2(g)��ƽ����ϵ�и��������������±���
| ���� | CH4 | CO2 | CO | H2 |
| ������� | 0.1 | 0.1 | 0.4 | 0.4 |
 2CO(g)��2H2(g) �ġ�H= ��
2CO(g)��2H2(g) �ġ�H= ��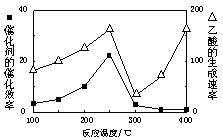
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ֱ�Ӽ״�ȼ�ϵ�أ�DNFC������Ϊ��21���͵綯������Ѻ�ѡ����Դ��
��1��101 kPaʱ��1 mol CH3OH��ȫȼ�������ȶ���������ų�����726.51 kJ/mol����״�ȼ�յ��Ȼ�ѧ����ʽΪ���� ������ ��
��2���״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧԭ���ǣ�
��CH3OH(g)+H2O(g)=CO2(g)+3H2(g) ����H1="+49.0" kJ��mol-1
��CH3OH(g)+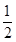 O2(g)= CO2(g)+2H2(g)����H2
O2(g)= CO2(g)+2H2(g)����H2
��֪H2(g)+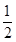 O2(g)=H2O(g)������H ="-241.8" kJ��mol-1
O2(g)=H2O(g)������H ="-241.8" kJ��mol-1
��Ӧ�ڵġ�H2= ��������������������������kJ��mol-1��
��3���״�ȼ�ϵ�صĽṹʾ��ͼ���ҡ��״������� ��������������������������ĵ缫��ӦʽΪ���������� ������ ���������������������ĵ缫��ӦʽΪ���������� �� ����������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
Ϊ��СCO2�Ի�����Ӱ�죬���������ŷ�����ͬʱ��Ӧ��ǿ��CO2�������õ��о���
��1���ٰѺ��нϸ�Ũ��CO2�Ŀ���ͨ�뱥��K2CO3��Һ�����ڢٵ�����Һ��ͨ����ˮ�����õ���Ũ�ȵ�CO2���塣д�����з�Ӧ�Ļ�ѧ����ʽ__________________________��
��2���罫CO2��H2��1:3������Ȼ�ϡ�
���ʵ������ºϳ�ij����ˮ�������� ������ţ���
| A������ | B��ϩ�� | C��Ȳ�� | D������ͬϵ�� |
 CH3OH(g)+H2O(g) ��H=��49.0 kJ/mol�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
CH3OH(g)+H2O(g) ��H=��49.0 kJ/mol�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
| �ܽ�ȣ�S��/g | �ܶȻ���Ksp�� | ||
| Ca(OH)2 | Ba(OH)2 | CaCO3 | BaCO3 |
| 0.16 | 3.89 | 2.9��10��9 | 2.6��10��9 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com