
���� O2��CO��H2��Ӧ�ķ���ʽ�ֱ�ΪO2+2CO$\frac{\underline{\;��ȼ\;}}{\;}$2CO2��O2+2H2$\frac{\underline{\;��ȼ\;}}{\;}$2H2O�����ݷ���ʽ֪��ǰһ������ʽ�����������Ϊԭ����$\frac{2}{3}$����һ������ʽ��������û�����壻
��1����ʣ������������15L���������������10L����CO��H2������ֱ���xL��yL��
�����$\left\{\begin{array}{l}{x+y=10}\\{0.5x+1.5y=10}\end{array}\right.$�����ݷ���ʽ������������������
��2����ʣ����������ΪaL���������������=��15+10-a��L��
����CO���������֮�ͼ���������������з���ʽ����㣻
��3����ʣ����������ΪaL�����ü�������a�ķ�Χ�����������ȫ��COʱ��ʣ���������������������ȫ��������ʣ�����������С��
��� �⣺O2��CO��H2��Ӧ�ķ���ʽ�ֱ�ΪO2+2CO$\frac{\underline{\;��ȼ\;}}{\;}$2CO2��O2+2H2$\frac{\underline{\;��ȼ\;}}{\;}$2H2O�����ݷ���ʽ֪��ǰһ������ʽ�����������Ϊԭ����$\frac{2}{3}$����һ������ʽ��������û�����壻
��1����ʣ������������15L���������������10L����CO��H2������ֱ���xL��yL��
�����$\left\{\begin{array}{l}{x+y=10}\\{0.5x+1.5y=10}\end{array}\right.$����$\left\{\begin{array}{l}{x=5}\\{y=5}\end{array}\right.$��
�ʴ�Ϊ��5��5��
��2����ʣ����������ΪaL���������������=��15+10-a��L����CO��H2������ֱ���xL��yL��
$\left\{\begin{array}{l}{x+y=10}\\{0.5x+1.5y=25-a}\end{array}\right.$��
���$\left\{\begin{array}{l}{x=a-10}\\{y=20-a}\end{array}\right.$��
V��CO����V��H2��=$\frac{a-10}{20-a}$��
�ʴ�Ϊ��$\frac{a-10}{20-a}$��
��3����ʣ����������ΪaL�����ü�������a�ķ�Χ�����������ȫ��COʱ��ʣ���������������������ȫ��������ʣ�����������С��
�����ȫ��CO����������ٵ����Ϊ5L��ʣ���������Ϊ20L��
�����ȫ����������������ٵ����Ϊ15L��ʣ���������Ϊ10L��
��a��ȡֵ��ΧΪ10L��20L��
�ʴ�Ϊ��10L��20L��
���� ���⿼�黯ѧ����ʽ���йؼ��㣬Ϊ��Ƶ���㣬��ȷ����������֮��Ĺ�ϵʽ�ǽⱾ��ؼ������ؿ���ѧ������������������3������ü�ֵ�����������Ŀ�ѶȲ���


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ˮ$\stackrel{NaOH}{��}$Mg��OH��2$\stackrel{���}{��}$Mg | |
| B�� | ��ˮ$\stackrel{HCl}{��}$MgCl2��Һ��MgCl2����$\stackrel{���}{��}$Mg | |
| C�� | ��ˮ$\stackrel{ʯ����}{��}$Mg��OH��2$\stackrel{����}{��}$MgO$\stackrel{���}{��}$Mg | |
| D�� | ��ˮ$\stackrel{ʯ����}{��}$Mg��OH��2$\stackrel{HCl}{��}$MgCl2��Һ-��MgCl2���壨���ڣ�$\stackrel{���}{��}$Mg |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ��������������ȷ���ǣ�������
��������������ȷ���ǣ�������| A�� | ���Ӽ䲻���γ���� | B�� | �����м��м��Լ����зǼ��Լ� | ||
| C�� | ��������7���Ҽ���1���м� | D�� | �÷�����ˮ�е��ܽ�ȴ���2-��ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | p-p | B�� | sp-p | C�� | sp2-p | D�� | sp2-s |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | �������ǻ�����ԭ�ӡ������������ǹ����� | |
| B�� | �ڶ��ױ�������ͬ���칹�壬˵�������������е�̼̼��������ȫ��ͬ�� | |
| C�� | ��ϩ��������������ԭ�ӹ�ƽ�� | |
| D�� | CH2Cl2������ͬ���칹�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ԭ��������42 | B�� | ��������14 | C�� | ��������42 | D�� | ��������14 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH2=CH2+Br2��CH2BrCH2Br | B�� | 2CH3CH3+Cl2$\stackrel{����}{��}$2CH3CH2Cl+H2 | ||
| C�� | CH3CH2OH+Na��CH3CH2ONa+H2�� | D�� | 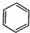 +Br2$\stackrel{FeBr_{3}}{��}$ +Br2$\stackrel{FeBr_{3}}{��}$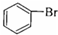 +HBr +HBr |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���Ӱ뾶 | ��һ������ | �۵� | ���� |
| O2-��Na+ | Si��S | NaCl��NaF | HClO4��H2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
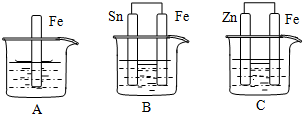
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com