
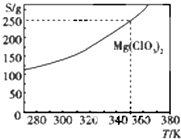
 Mg��ClO3��2��ũҵ�Ͽ�������Ҷ������������ɲ��ø��ֽⷴӦ�Ʊ���MgCl2+2NaClO3�TMg��ClO3��2+2NaCl����֪Mg��ClO3��2���ܽ�ȣ�S�����¶ȣ�T���ı仯������ͼ��ʾ�������й������в���ȷ���ǣ�������
Mg��ClO3��2��ũҵ�Ͽ�������Ҷ������������ɲ��ø��ֽⷴӦ�Ʊ���MgCl2+2NaClO3�TMg��ClO3��2+2NaCl����֪Mg��ClO3��2���ܽ�ȣ�S�����¶ȣ�T���ı仯������ͼ��ʾ�������й������в���ȷ���ǣ�������| A�� | �¶�Խ�ߣ�Mg��ClO3��2������Һ�����ʵ���Ũ��Խ�� | |
| B�� | �¶�Խ�ߣ�Mg��ClO3��2������Һ����������Խ�� | |
| C�� | 350Kʱ��Mg��ClO3��2������Һ�����ʵ���Ũ��Ϊ$\frac{250g}{191g/mol��1L}$ | |
| D�� | 350 Kʱ��Mg��ClO3��2������Һ����������Ϊ$\frac{250g}{350g}$��100% |
���� A����ͼ���֪�������¶ȵ����ߣ�Mg��ClO3��2���ܽ������
B����ͼ���֪�������¶ȵ����ߣ�Mg��ClO3��2���ܽ������
C����Һ�������֪�����������ʵ���Ũ�ȣ�
D������$\frac{���ʵ�����}{��Һ������}$��100%���㣮
��� �⣺A����ͼ���֪�������¶ȵ����ߣ�Mg��ClO3��2���ܽ���������¶�Խ�ߣ�Mg��ClO3��2������Һ�����ʵ���Ũ��Խ��A��ȷ��
B����ͼ���֪�������¶ȵ����ߣ�Mg��ClO3��2���ܽ���������¶�Խ�ߣ�Mg��ClO3��������Һ����������Խ��B��ȷ��
C��350Kʱ��Mg��ClO3��2������Һ���ܽ��Ϊ250g��������Һ�������֪�����������ʵ���Ũ�ȣ���C����
D��350Kʱ��Mg��ClO3��2������Һ���ܽ��Ϊ250g����100gˮ�����ʵ�����Ϊ250g������Һ����������Ϊ$\frac{���ʵ�����}{��Һ������}$��100%=$\frac{250g}{350g}$��100%����D��ȷ��
��ѡC��
���� ���⿼�����ܽ�ȵĺ��塢���ʵ���Ũ�ȡ���Һ���������ļ���ȣ���Ŀ�ѶȲ������ڿ���ѧ���ķ��������Ͷ�ͼ������


 ����ѧ����ϵ�д�
����ѧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �ڢ� | C�� | �٢� | D�� | �ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��1����һ���Ϊ10L�������У�ͨ��һ������CO��H2O����850��ʱ�������·�Ӧ��
��1����һ���Ϊ10L�������У�ͨ��һ������CO��H2O����850��ʱ�������·�Ӧ��| ʱ�䣨min�� | 0 | 2 | 3 | 4 | 5 | 6 |
| CO | 0.200 | 0.138 | C1 | C1 | 0.116 | 0.096 |
| H2O | 0.300 | 0.238 | C2 | C2 | 0.216 | 0.266 |
| CO2 | 0 | 0.062 | C3 | C3 | 0.084 | |
| H2 | 0 | 0.062 | C3 | C3 | 0.104 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��������͵����������dz��õĹ�ҵԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�����ѧ��ǰ����Ҫ�о�����֮һ��
��������͵����������dz��õĹ�ҵԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�����ѧ��ǰ����Ҫ�о�����֮һ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | n��Cl2����n��Fe��=5��4 5Cl2+4Fe $\frac{\underline{\;��ȼ\;}}{\;}$2FeCl2+2FeCl3 | |
| B�� | n��Cl2����n��FeBr2��=1��1 Fe2++2Br-+Cl2�TFe3++Br2+2Cl- | |
| C�� | n��MnO4-����n��H2O2��=2��3 2MnO4-+3H2O2+6H+�T2Mn2++4O2��+6H2O | |
| D�� | n��Fe����n[HNO3��ϡ��]=1��3 4Fe+12H++3NO3-�T3Fe2++Fe3++3NO��+6H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

 ��
�� �����ͬ���칹��Ľṹ��ʽΪ��
�����ͬ���칹��Ľṹ��ʽΪ�� ��
�� ���������ͪ��������������·���з�Ӧ�١���֮�õ����л�����Ľṹ��ʽΪ��
���������ͪ��������������·���з�Ӧ�١���֮�õ����л�����Ľṹ��ʽΪ�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | O2��O3��Ϊͬ�������� | |
| B�� | O3����3����ԭ�ӹ��ɵĻ����� | |
| C�� | O2��O3�ת�����ڻ�ѧ�仯 | |
| D�� | ��������O2��O3���е���ԭ������ͬ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com