

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| X |
| X |
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
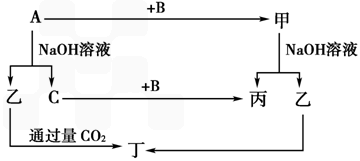
 ��֪A��B��C����ѧ��ѧ�ij������ʣ�������һ������������ͼ��ʾת����ϵ��
��֪A��B��C����ѧ��ѧ�ij������ʣ�������һ������������ͼ��ʾת����ϵ��
| ||
| ||
| ||
| �� |
| ||
| �� |


| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�022
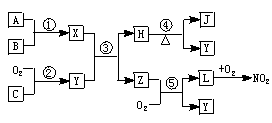
��������ϲ��ϣ��ش��������⣺
(1)�û�ѧʽ��ʾ������L_______������C______��
(2)����Z�ķ��ӿռ乹����______��
(3)��Ӧ���Ļ�ѧ����ʽ______��
(4)��Ӧ���Ļ�ѧ����ʽ______��
(5)�ڳ������������ܶȲⶨNO2����Է���������ʵ��ֵ������ֵƫ_____(����������������)����ԭ����___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
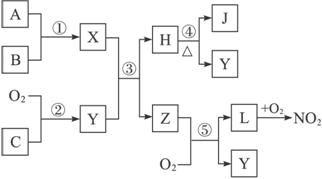
��1���û�ѧʽ��ʾ������L��_____________������C��_____________��
��2������Z�ķ��ӿռ�ṹ��_____________��
��3����Ӧ�ٵĻ�ѧ����ʽΪ_____________________________________________��
��4����Ӧ�ݵĻ�ѧ����ʽΪ_____________________________________________��
��5���ڳ������������ܶȲⶨNO2����Է���������ʵ��ֵ������ֵƫ����ߡ��͡�������ԭ����____________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com