
����Ŀ����������X��Y��Z����Է���������ϵΪMr(X) < Mr(Y)= 0.5Mr(Z),����˵����ȷ����()
A.ԭ����Ŀ��ȵ���������,����������Z
B.��ͬ������,ͬ��������������,�����ܶ���С����X
C.��һ��������,�������������Ϊ2.24 L,�����ǵ����ʵ���һ����Ϊ0.1 mol
D.ͬ����,�����ͬ���������ֱ��2gY�����1gZ����,����ѹǿ��Ϊ2��1
���𰸡�B
��������
��������X��Y��Z����Է���������ϵΪMr(X) < Mr(Y)= 0.5Mr(Z)����Mr(X) < Mr(Y) < Mr(Z)��
Aѡ�����![]() ��������Ŀ��ȵ��������壬������������Է�����������Z��ԭ����Ŀ��ȵ��������壬�����������жϣ���A����
��������Ŀ��ȵ��������壬������������Է�����������Z��ԭ����Ŀ��ȵ��������壬�����������жϣ���A����
Bѡ������ܶ�![]() �����崦����ͬ�������£����ܶȺ���Է������������ȣ����������ܶ���С����X����B��ȷ��
�����崦����ͬ�������£����ܶȺ���Է������������ȣ����������ܶ���С����X����B��ȷ��
Cѡ���������ʵ���![]() ��Vm�������״̬�йأ��������������Ϊ2.24 L,
��Vm�������״̬�йأ��������������Ϊ2.24 L,
Vm��һ������22.4 L/mol���������ǵ����ʵ�����һ����Ϊ0.1 mol����C����
Dѡ�ͬ���£������ͬ���������ֱ��2gY�����1gZ���壬Mr(Y)= 0.5Mr(Z)������ߵ����ʵ���֮����4��1��ͬ��ͬ������������ʵ���֮�ȵ���ѹǿ֮�ȣ�Y��Z���������ܵ�ѹǿ��Ϊ4��1����D����
������������ΪB��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��KMnO4��ʵ�����г��õ�һ���Լ����ش��������⣺
��1��K+�Ľṹʾ��ͼΪ___��
��2�������Ը��������Һ�еμӹ����IJ���(H2C2O4������)��Һ������Һ��ɫ����ɫ�������Ļ�ѧ��ӦΪ2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+10CO2��+8H2O�������÷�Ӧ��Ƴ�ԭ��أ�����ӦʽΪ___��
��3����10mL0.1mol��L-1KMnO4��Һ(��ϡ����)�м���15mL0.5mol.L-1������Һ���ռ�����CO2������ʱ��Ĺ�ϵ��ͼ��ʾ��

AB�η�Ӧ���������ԭ�������___(����ĸ)��
a.�÷�Ӧ�Ƿ��ȷ�Ӧ
b.��Ӧ��Ũ������
c.K2SO4�������
d.MnSO4�������
��4��Ϊ��̽����������Ի�ѧ��Ӧ���ʵ�Ӱ�죬������·�����
ʵ�� | 0.1mol��L-1KMnO4/mL | 0.5mol��L-1H2C2O4/mL | 0.1mol��L-1H2SO4/mL | ˮԡ �¶�/�� | ����ˮ /mL | ��ɫʱ�� /min |
I | 5.0 | 15.0 | 5.0 | 35 | 0 | t1 |
II | 5.0 | 10.0 | 5.0 | 35 | V | t2 |
III | 5.0 | 15.0 | 3.0 | 35 | 2.0 | t3 |
IV | 5.0 | 15.0 | 5.0 | 45 | 0 | t4 |
��V=___��
�ڸ�ʵ�鷽������̽���Է�Ӧ������Ӱ���������___��
A.Ũ�� B.��� C.�¶� D.����
������ý����t4��t1����ʵ�������___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧС���ڲ�������ʵ��̽���Ļ����ϣ���̵���ʶ��±�ص����ʡ�
I��F2��C12�������ԡ�1971��N��H��Studier��E��H��Appelman�ӱ�(��40��)�ķ����������״η���F2��C12��ˮ�ķ�Ӧ���ơ�
(1)д��������ˮ����������Ӧ�Ļ�ѧ����ʽ_____________________________________��
II���������ƺ�����ص��Ʊ�����ͼװ�����������װ�õ������ԡ�

��һ��������ƿ�ڷ�Լ3g MnO2��ĩ����ȫ©�������Թܵײ���
�ڶ������ڹ�6�з�4mL 6mol��L-1KOH��Һ(����ˮԡ��)����7�з�4mL 2mol�� L-1NaOH��Һ(�ű�ˮԡ��)�����Ƽ�3���رտ��Ƽ�4��
����������©������15mL 9mol��L-1HC1��Һ���������ȣ������������Ȳ�������ˮԡ�¶ȿ�����323K��328K��
���IJ���һ��ʱ���ֹͣ���ȣ�����
(2)��ƿ����С�Թܵ��ŵ�Ϊ_________________________________________________��
(3)ֹͣ���Ⱥ�IJ�����____________________________________���ٽ���6��7���¡�
(4)������������֪������NaC1O��Һ���ȣ�NaC1O�ֽ������NaC1O3���ݴ��ƶ�����KC1O3������673K����ֽ�Ļ�ѧ����ʽΪ_____________________________________��
��C1-��Br-��I-�Ļ��Һ��C1-�ļ���
��֪��Ksp(AgC1)=1.8��10-10 Ksp(AgBr)=5.4��10-13 Ksp(AgI)=8.5��10-17

��һ����ȡ2-3��C1-��Br-��I-�Ļ��Һ����1��6mol��L-1HNO3��Һ�ữ���μ�0.1mol��L-1AgNO3��Һ��������ȫ������2min�����ķ��룬��ȥ��Һ��
�ڶ������ڳ����м���5��10��2mol��L-1NH3��H2O��Һ�����ҽ��裬������1min�����ij���������Һ����һֻ�Թ��С�
(5)��֪![]() ����ƽ��ʱ[Ag(NH3)2]+Ũ��Ϊ0.1mol��L-1�����ܽ�AgBr�������谱ˮ�����Ũ��ԼΪ_______mol��L-1(
����ƽ��ʱ[Ag(NH3)2]+Ũ��Ϊ0.1mol��L-1�����ܽ�AgBr�������谱ˮ�����Ũ��ԼΪ_______mol��L-1(![]() )��
)��
(6)�����������㣬����֪��Һ��������Ҫ�ɷֵĻ�ѧʽΪ_________________________��
����������Һ��6mol��L-1HNO3�ữ��
(7)������_______________________________________________��֤ʵC1-���ڡ���Ӧ�����ӷ���ʽΪ__________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ�У�������ȡ����Ӧ����
A. ![]() +HNO3
+HNO3![]()
![]() +H2O
+H2O
B. CH2=CH2+Br2 ![]() BrCH2CH2Br
BrCH2CH2Br
C. CH4+Cl2![]() CH3Cl+HCl
CH3Cl+HCl
D. CH3CH2CH2Br+NaOH![]() CH3CH2CH2OH+NaBr
CH3CH2CH2OH+NaBr
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������NO2��O2�Ļ������12 mL��ͨ������ˮ�У���ַ�Ӧ��ʣ������2 mL(ͬ��ͬѹ��)����ԭ��������������������(����)
A.1.3 mLB.2.4 mLC.3 mLD.4 mL��1.2 mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ���Թ���ʢװ���Ǻ���ɫ���壨�����ǻ�������������ʢ��ˮ��ˮ����ʱ���Թ���Һ�������������ܳ����Թܣ������Թ��ڹ����������Թ۲쵽�Թ���Һ�������������������ظ����Թ�����ȫ����Һ�壬ԭ���Թ���ʢװ�������ǣ� ��
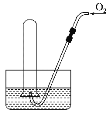
A.������N2��NO2�Ļ������
B.һ����NO2����
C.������NO��NO2�Ļ������
D.ֻ������NO2һ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijС�����������װ�ý���ʵ�飬�ڡ�������Һ���������������������±���

ʵ�� | ���� | ���� |
�� | ��ʢ��Na2S��Һ�Ģ��г���ͨ��CO2������ | ���в�����ɫ��������Һ��pH���ͣ� ���в�����ɫ���ǣ��û�������ð���� |
�� | ��ʢ��NaHCO3��Һ�Ģ��г���ͨ��H2S���������� | ����ͬʵ��� |
���ϣ�CaS��ˮ��ȫˮ��
������ʵ��ó��Ľ�������ȷ����
A. ���а�ɫ������CaCO3
B. ������ҺpH���͵�ԭ���ǣ�H2S+Cu2+ == CuS��+2H+
C. ʵ������CO2���������ķ�Ӧ�ǣ�CO2+H2O+ S2== CO32+ H2S
D. ��ʵ���͢��ܱȽ�H2CO3��H2S���Ե�ǿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ�����ײ��������������к���ֲ�����������ȫ�����֣���������������������Ŀ���Ԫ����ʹ����NH4NO3��KNO3��CaCl2��2H2O��MgSO4��7H2O����������Һ����Ԫ����Һ����ȱ����һ�ֱ���Ԫ�أ�Ϊ��������Ԫ�أ�Ӧ���ӵĻ������ǣ� ��

A.Ca��NO3��2B.KClC.KH2PO4D.K2SO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ó�Ϊ�����о����ȵ㣬�����͵���з�����Ҫ�IJ��ϡ�
��1��ͨ�����·�Ӧ�Ʊ���������
��Ӧ1��Al2O3(s)��AlCl3(g)��3C(s)===3AlCl(g)��3CO(g)����H1��akJ��mol��1
��Ӧ2��Al2O3(s)��3C(s)===2Al(l)��3CO(g)����H2��bkJ��mol��1
��Ӧ3��3AlCl(g)===2Al(l)��AlCl3(g)����H3
�ٷ�Ӧ3����H3��_______kJ��mol��1��
��950��ʱ���������������Ľ�̿��Cl2��Ӧ���Ƶ�AlCl3���÷�Ӧ�Ļ�ѧ����ʽ��_______��
��2���ڸ��������½��з�Ӧ��2Al(l)��AlCl3(g)![]() 3AlCl(g)��
3AlCl(g)��
����ͼ1��ʾ�ĵ��ݻ�A��B�ܱ������м���������Al�ۣ��ٷֱ����1 mol AlCl3(g)������ͬ�ĸ����½��з�Ӧ��ͼ2��ʾA�����ڵ�AlCl3(g)���������ʱ��ı仯ͼ����ͼ2�л���B������AlCl3(g)���������ʱ��ı仯���ߡ�__________
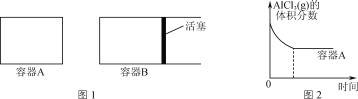
��1100��ʱ����2 L�ܱ�������ͨ��3 mol AlCl(g)��������Ӧ��3AlCl(g)=2Al(l)��AlCl3(g)����֪���¶���AlCl(g)��ƽ��ת����Ϊ80%����÷�Ӧ��ƽ�ⳣ��K��________��
�ۼ���3molAlCl(g)���ڲ�ͬѹǿ�·�����Ӧ���¶ȶԲ��ʵ�Ӱ����ͼ3��ʾ���˷�Ӧѡ���¶�Ϊ900���ԭ����_______________��
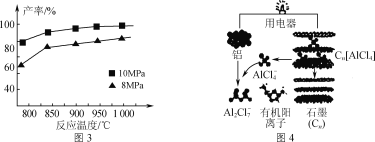
��3�����������Ŀ��ٷŵ������Ӷ��ε�ص�ԭ����ͼ4��ʾ��
�ٸõ�س��ʱ�������ĵ缫��ӦʽΪ_____��
��AlCl3��NaCl�������γ����ڶ������أ����ʱAlCl4-��Al2Cl7-���������ڵ缫���ת�����������Ӳ�����缫��Ӧ��NaCl��������_____��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com