
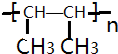 ��
��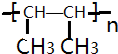 ���Ӿ۷�Ӧ��
���Ӿ۷�Ӧ��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
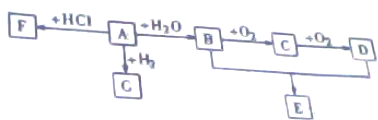
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A�� ֤���ǽ�����ǿ����S��C��Si |
B�� �Ʊ��������������Ʒ�Ӧ |
C�� �Ʊ����ռ�����NO���� |
D�� ��ȡ0.10 mol?L-11KOH��Һ20.00 mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ö������������������Һ�͵�����Һ |
| B����ȼ�ŵ�ľ������CO2��O2 |
| C����ϡ�������пƬ��ͭƬ |
| D���ü�ˮ�ܽ�ķ������ɼ���ʳ�κͰ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij�¶��£����ڷ�ӦN2��g��+3H2��g��?NH3��g������H=-92.4kJ/mol��N2��ƽ��ת���ʣ���������ϵ��ѹǿ��P���Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
ij�¶��£����ڷ�ӦN2��g��+3H2��g��?NH3��g������H=-92.4kJ/mol��N2��ƽ��ת���ʣ���������ϵ��ѹǿ��P���Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A����1.0mol������3.0mol����������1L�ܱ������з�����Ӧ���ų�������Ϊ92.4kJ |
| B��ƽ��״̬��A�䵽Bʱ��ƽ�ⳣ��K��A����K��B�� |
| C��������Ӧ�ڴﵽƽ�������ѹǿ��H2��ת������� |
| D������ѹǿ���䣬ͨ��������壬ƽ�ⳣ�����䣬ƽ�ⲻ�ƶ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����1mol������ˮ��Һ�м�����������Һ�����������ﵽ���ֵ�����������ʵ���Ϊ2mol |
| B���������ữ�ĸ��������˫��ˮ��ϣ�����֤���������л�ԭ�ԣ������ӷ���ʽΪ�� 2MnO4-+5H2O2+6H+=4Mn2++8H2O+5O2�� |
| C��ʹ���������Զ�ˮ����������ɱ�� |
| D���������׳�ˮ�������ݻ������ճ�ϼ����ͻ���ȼ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������������ˮ��Ӧ������������������ |
| B������ˮ���������·�Ӧ�������������� |
| C������������������+3�ۣ�����-1�� |
| D���������������ǿ��ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ������ | ��Ba2+��Ag+��Mg2+��Na+ |
| ������ | SO42-��SO32-��CO32-��AlO2- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��KMnO4 |
| B��Cl2 |
| C��HCl |
| D��FeCl3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com