
| �¶ȣ��棩 | 10 | 20 | 30 | 50 | 70 |
| pH | 8.3 | 8.4 | 8.5 | 8.9 | 9.4 |



 ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д�
ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д� Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
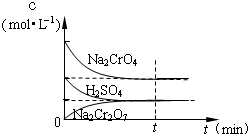 ��25��ʱ����Na2CrO4��Һ�еμ�ϡ���ᣬ��Һ�ɻ�ɫת��Ϊ��ɫ���ڴ�ת�������У�������Ũ�ȱ仯��ͼ��ʾ��д��ת�������з�����Ӧ�����ӷ���ʽ
��25��ʱ����Na2CrO4��Һ�еμ�ϡ���ᣬ��Һ�ɻ�ɫת��Ϊ��ɫ���ڴ�ת�������У�������Ũ�ȱ仯��ͼ��ʾ��д��ת�������з�����Ӧ�����ӷ���ʽ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08�Ϻ�12У�ڶ�����������A������������ȩ�������������������ʵ�飬
��ͬѧ�������ͼʵ��װ�ý��У���B�Թ��м���
10ml 40%����ȩˮ��Һ��5g�����̾���ͷ�ʯ�Ļ�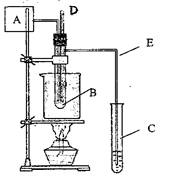
������ձ��м�50ml�Ĺ��ͣ���C�Թ��м���
10ml����ˮ��
��֪������ʵķе����±���
���� | ��ȩ | ���� | ���� | ˮ | ֲ���� |
�е� | 20.8�� | 117.9�� | 180������ | 100�� | 175�� |
���¶ȼƵĶ���Ϊ60��D80��ʱ��A���������10�D15�Σ�
�������ȣ��������Թ�C�еõ���ˮ��ҺΪ������Һ����ش��������⣺
��1��д������������ȡ����Ļ�ѧ����ʽ ��
��2�����ձ��зŹ��͵�ԭ���� ��
Ϊ�˱�֤ʵ��ijɹ������ձ���Ҳ������ ������͡��ڹ������ǰ�� ���¶ȼ�ˮ�����λ���ǣ�����ǰ ����� ������¶�Ӧ������ ��
��3����C�Թ����ռ�����ˮ��Һ����ij��������ǣ�
�� ��
�� ��
�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08�Ϻ�12У�ڶ�����������A������������ȩ�������������������ʵ�飬
��ͬѧ�������ͼʵ��װ�ý��У���B�Թ��м���
10ml 40%����ȩˮ��Һ��5g�����̾���ͷ�ʯ�Ļ�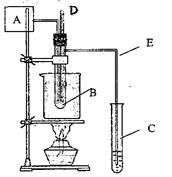
������ձ��м�50ml�Ĺ��ͣ���C�Թ��м���
10ml����ˮ��
��֪������ʵķе����±���
���� | ��ȩ | ���� | ���� | ˮ | ֲ���� |
�е� | 20.8�� | 117.9�� | 180������ | 100�� | 175�� |
���¶ȼƵĶ���Ϊ60��D80��ʱ��A���������10�D15�Σ�
�������ȣ��������Թ�C�еõ���ˮ��ҺΪ������Һ����ش��������⣺
��1��д������������ȡ����Ļ�ѧ����ʽ ��
��2�����ձ��зŹ��͵�ԭ���� ��
Ϊ�˱�֤ʵ��ijɹ������ձ���Ҳ������ ������͡��ڹ������ǰ�� ���¶ȼ�ˮ�����λ���ǣ�����ǰ ����� ������¶�Ӧ������ ��
��3����C�Թ����ռ�����ˮ��Һ����ij��������ǣ�
�� ��
�� ��
�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com