
| n |
| V |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
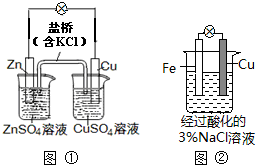
| A��ͼ��������������ҺpH���� |
| B��ͼ���е�����Zn����Cu�������е�Cl-����CuSO4��Һ |
| C��ͼ��������Ӧ��O2+2H2O+4e-�T4OH- |
| D��ͼ���м�������K3[Fe��CN��6]��Һ������ɫ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
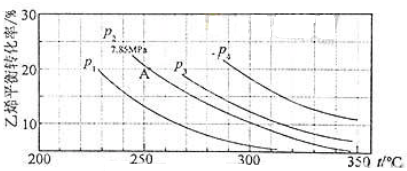
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
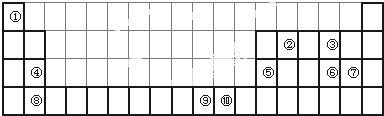
 �����и߶ȶԳ��ԡ��������ԡ��������ܼ����ȶ��Ե��ص㣬��˺ϳ������鼰���������Ϊ��ѧ���ע���ȵ㣮������������������I��һ�ֺϳ�·�ߣ�
�����и߶ȶԳ��ԡ��������ԡ��������ܼ����ȶ��Ե��ص㣬��˺ϳ������鼰���������Ϊ��ѧ���ע���ȵ㣮������������������I��һ�ֺϳ�·�ߣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
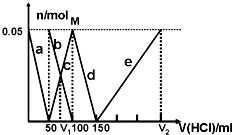 ijNa2CO3��NaAlO2�Ļ����Һ����μ���1mol?L-1�����ᣬ�����Һ�е�CO32-��HCO3-��AlO2-��Al3+���ӵ����ʵ��������������Һ������仯��ϵ��ͼ��ʾ��������˵������ȷ���ǣ�������
ijNa2CO3��NaAlO2�Ļ����Һ����μ���1mol?L-1�����ᣬ�����Һ�е�CO32-��HCO3-��AlO2-��Al3+���ӵ����ʵ��������������Һ������仯��ϵ��ͼ��ʾ��������˵������ȷ���ǣ�������| A��ԭ�����Һ�е�CO32-��AlO2-�����ʵ���֮��Ϊ1��2 |
| B��V1��V2=1��4 |
| C��M��ʱ���ɵ�CO2Ϊ0mol |
| D��a���߱�ʾ�����ӷ���ʽΪ��AlO2-+H++H2O�TAl��OH��3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| c(Cl-) |
| c(ClO-) |
A����ij�¶��£���Ӧ��
| ||||||
B���μӷ�Ӧ�����������ʵ�������
| ||||||
C���ı��¶ȣ���Ӧ��ת�Ƶ��ӵ����ʵ���ne�ķ�Χ��
| ||||||
D���ı��¶ȣ�������KClO3��������۲���Ϊ
|
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com