
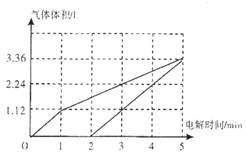
 ����֪���¶ȹ���ʱ��WO2��s��ת��ΪWO2��g����
����֪���¶ȹ���ʱ��WO2��s��ת��ΪWO2��g����
| ||
| 2min |
| 2a+18b |
| a+b |
| c4(H2O) |
| c4(H2) |
(
| ||
(
|
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
| ||
| 0.1mol |
| 1L |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ɫ��Ⱦ | B��������Ⱦ |
| C��������Ⱦ | D����������Ⱦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������ʹ�ÿ�ʵ��SO2��ת����Ϊ100% |
| B���ﵽ��ѧƽ��ʱ�������ʵ�Ũ�Ȳ��ٸı� |
| C�������������䣬�����¶ȣ���������Ӧ������ |
| D�������������䣬����SO3��Ũ�ȣ���������Ӧ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��CH3-CH2-CH3��CH3-CH2-CH2-CH3 |
B��CH3һCH=CH-CH3�� |
| C��CH3-CH=CH-CH3��CH3-CH2-CH=CH2 |
D�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� | ||||||||
| �� | �� | �� | �� | |||||
| �� | �� | �� | �� | |||||
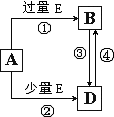
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �¶�/�� | 300 | 400 | 500 | 750 | 1000 |
| ƽ�ⳣ�� | 1.8 | 1.6 | 1.0 | 0.8 | 0.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 Ϊ����������ʣ���������ȱ��Ӱ����������������ҹ����� ��ȡʳ�μӵ��ʩ���ݱ������˴�ʳ������ȡ������ڼ�״���л���������ͨ��һϵ�л�ѧ��Ӧ���γɼ�״���أ���״���صĽṹ��ͼ���ش��������⣺
Ϊ����������ʣ���������ȱ��Ӱ����������������ҹ����� ��ȡʳ�μӵ��ʩ���ݱ������˴�ʳ������ȡ������ڼ�״���л���������ͨ��һϵ�л�ѧ��Ӧ���γɼ�״���أ���״���صĽṹ��ͼ���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com