
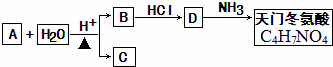
 ��
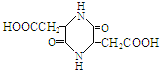
�� ���� A��������������ˮ���B��C����֪AΪ������ͨ��״����BΪ��ɫ���壬��������������Һ������Ӧ����BΪ���ᣬC���ڴ�������B�ķ���ʽΪC4H4O4����֪ÿ����B�к�2��-COOH�����A������ˮ������ʹ���CCl4��Һ��ɫ��˵��A�����к���̼̼�����ͼ�����B�г����Ȼ��⣬����C=C������ϣ�3����B����û��֧������BΪHOOCCH=CHCOOH��C��һ��ͬϵ��������㷺ʹ�õ����ϳɷ֣���CΪCH3OH����AΪCH3OOCCH=CHCOOCH3��B��HCl�����ӳɷ�Ӧ����CΪHOOCCH2CHClCOOH�������Ŷ�����Ľṹ��ʽ��HOOCCH2CH��NH2��COOH���Դ˽����⣮
��� �⣺A��������������ˮ���B��C����֪AΪ������ͨ��״����BΪ��ɫ���壬��������������Һ������Ӧ����BΪ���ᣬC���ڴ�������B�ķ���ʽΪC4H4O4����֪ÿ����B�к�2��-COOH�����A������ˮ������ʹ���CCl4��Һ��ɫ��˵��A�����к���̼̼�����ͼ�����B�г����Ȼ��⣬����C=C������ϣ�3����B����û��֧������BΪHOOCCH=CHCOOH��C��һ��ͬϵ��������㷺ʹ�õ����ϳɷ֣���CΪCH3OH����AΪCH3OOCCH=CHCOOCH3��B��HCl�����ӳɷ�Ӧ����CΪHOOCCH2CHClCOOH�������Ŷ�����Ľṹ��ʽ��HOOCCH2CH��NH2��COOH��
��1��AΪCH3OOCCH=CHCOOCH3�����е�̼̼˫���ܷ����ӳɡ��Ӿۡ������ȷ�Ӧ�����ܷ���������Ӧ���ʴ�Ϊ���٢ۢܣ�
��2��BΪHOOCCH=CHCOOH�����еĹ�����Ϊ̼̼˫�����Ȼ����ʴ�Ϊ��̼̼˫�����Ȼ���
��3��������������֪��B�Ľṹ��ʽ�ǣ�HOOCCH=CHCOOH���ʴ�Ϊ��HOOCCH=CHCOOH��
��4�������Ϸ�����֪�����Ŷ�����Ľṹ��ʽ�ǣ�HOOCCH2CH��NH2��COOH���ʴ�Ϊ��HOOCCH2CH��NH2��COOH��
��5�����������Ŷ�����һ�������¿�����������һ����Ԫ���Ľṹ�����ʣ��÷�Ӧ�Ļ�ѧ����ʽΪ��2HOOCCH2CH��NH2��COOH $\stackrel{һ������}{��}$2H2O+ ��
��
�ʴ�Ϊ��2HOOCCH2CH��NH2��COOH $\stackrel{һ������}{��}$2H2O+ ��
��
���� ���⿼���л�����ƶϣ�Ϊ��Ƶ���㣬������ѧ���ķ��������Ŀ��飬�ؼ��Ǹ���A�����ʼ�B�ķ���ʽ���ṹ�ص�������ۺϷ���ȷ��B�Ľṹ��ʽ���������չ����ŵ�������ת������Ŀ�Ѷ��еȣ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 14 | B�� | 15 | C�� | 16 | D�� | 17 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ϩ�����顢��Ȳ���ױ� | B�� | ���ӡ����ᡢ����������Ҵ� | ||
| C�� | �����ơ��廯�ơ��ء��Ȼ��� | D�� | ��������������ϩ���Ҵ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ʵ������ | ʵ��Ŀ�� | |
| A | ��AlCl3��Һ�������� | �Ʊ�Al2O3 |
| B | �����ˮ������Һ�У����μ���NaOH��Һ������Cu��OH��2������ | ̽��ˮ�����Ļ�ԭ�� |
| C | ��ij��Һ�м���ϡ���ᣬ������������ͨ�����ʯ��ˮ | �������Һ���Ƿ���CO32- |
| D | ��H2O2��Һ�еμ�����FeCl3��Һ | ̽�������Ի�ѧ��Ӧ���ʵ�Ӱ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �� NaHCO3 ��Һ��һ�μ����ϡ���ᡢNaOH��Һ��AlCl3��Һ��NaAlO2 ��Һ | |
| B�� | ��������Լ�ƿ�ڱ��Ϻ�ɫ���ʿ���ϡ����ϴ�� | |
| C�� | ��ȥSO2 ������HCl������ͨ�뱥�͵�Na2SO3 ��Һ | |
| D�� | ��NaOH��Һ��μ��뱥��FeCl3��Һ��ȡFe��OH��3���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����£�ͨ����pH��ֽ�ⶨŨ��Ϊ0.1 mol•L-1 NaClO��Һ��0.1 mol•L-1 CH3COONa��Һ��pH���Ƚ�HClO��CH3COOH������ǿ�� | |
| B�� | ������ƿ������Һ������ʱ���ӿ̶��ߣ�������ҺŨ��ƫС | |
| C�� | �ø��������Һ�ζ�Na2SO3��Һ���յ㣺�������һ�θ��������Һ����Һǡ������ɫ��Ϊ��ɫ������Ӳ���ɫ | |
| D�� | �ⶨ�кͷ�Ӧ�ķ�Ӧ��ʱ��������������У������¶�ֵƫС |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 25��ʱ��pH=11 NaOH��Һ��pH=3 CH3COOH��ϣ�����Һ�����ԣ���������Һ������Ũ�ȿ���Ϊ��c��CH3COO-����c��H+����c��Na+����c��OH-�� | |
| B�� | ȡc��H+��=0.01mol•L-1������ʹ����100mL���ֱ�ϡ��2�����ٷֱ����0.03gп�ۣ�����ͬ�����³�ַ�Ӧ��������п��Ӧ�����ʴ� | |
| C�� | ��Ũ�����ữ��KMnO4��Һ��H2O2��Ӧ��֤��H2O2���л�ԭ�ԣ�2MnO$_4^-$+6H++5H2O2=2Mn2++5O2��+8H2O | |
| D�� | �������ʵ�����NaHC2O4��Na2C2O4����Һ�д��ڣ�2c��Na+��=3[c��HC2O4-��+c��C2O42-��+c��H2C2O4��] |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ϴ������ʱ����ʹ�ú���ϴ�·ۣ�������ˮ��ֲ������� | |
| B�� | ��2008��6��1������ȫ����Χ�ڽ�ֹ���������ۡ�ʹ�ó������Ϲ���� | |
| C�� | ��ú����������Һ���Ի�ýྻ��ȼ�� | |
| D�� | ���á���ɫ��ѧ�����գ����������Ѻ��ͻ�����ϵ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Fe��OH��3��������Fe��OH��3+3H+�TFe3++3H2O | |
| B�� | �� NaAlO2��Һ��ͨ�����������̼��AlO2-+CO2+2H2O�TAl��OH��3+HCO3- | |
| C�� | ��ǿ����Һ�д�������������������Ӧ���� Na2FeO4��3ClO-+2Fe��OH��3�T2FeO42-+3Cl-+4H+ | |
| D�� | ��������Һ�е����Ȼ�����Һ��2Al3++3S2-�TAl2S3�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com