
����ͭ�����׳ơ������������������������������й㷺��Ӧ�á�ij�����о�С���ͬѧ�ô�ͭ�ۣ�����̼�����ʣ�����������Ʊ�������;�������ⶨ�����нᾧˮ�ĺ�������Ƶ��������£�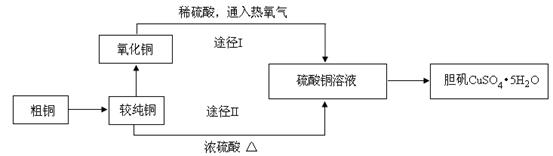
��1�����ϴ�ͭ��ת��Ϊ����ͭʱ��Ӧ�������� �ڽ������գ���д�������ƣ�������ͭ���������֬�������ȼ���Һϴȥ��ԭ���� �������ա���ͭ������õIJ����ǻ�������ͭ������ͭ����������ͭ�Ŀ���ԭ���� ��
a�����չ����в�������ͭ����ԭ b����������ͭ������������
c������ͭ�ڼ��ȹ����зֽ�����ͭ d�����ղ����ͭδ����ȫ����
��2��ͨ��;��Iʵ���ô�������ͭ��ȡ������������е�ʵ����������ǣ����ܡ�����ͨ���������ˡ� ����ȴ�ᾧ�� ����Ȼ����Ƚ��ɴ�������ͭ��ȡ����������;����;���������Ե������ŵ㣺
���������������������� ���� ��
�� ��
��3���ⶨ����������ᾧˮ�ĺ���ʱ�����ⶨ������������㣬���������ԭ�������___________��
a�����Ⱥ�����δ�������������ȴ
b��������μ��Ⱥ���������ϴ�
c������ǰ����ʱ����δ��ȫ����
d�����ȹ�������������ʧ
��4��������ͼװ�ü�����ˮ����ͭ��ĩֱ����ȫ�ֽ⣬A���Թ���ʣ���ɫ��ĩ���ô����ǵ�ľ�����뼯��ƿD������ľ���ܸ�ȼ����Ӧǰ���װ�õ�������ͼ�·��ı�����ʾ��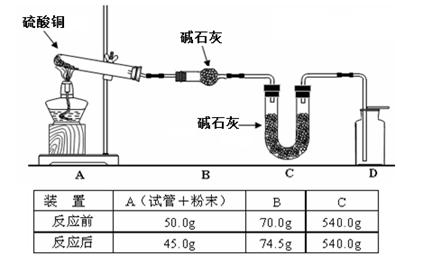
��ͨ�����㣬�ƶϸ�ʵ������������ͭ�ֽ�Ļ�ѧ����ʽ�� ��
��1����������֬�ڼ��������·���ˮ�����ȥ����2�֣���1�֣��� ad��2�֣���1�֣�
��2������Ũ�� ����ϴ�ӣ�2�֣���1�֣����ٲ�������������;��I���������٣���;��I��������Ⱦ���������壨2�֣���1�֣�
��3��cd��2�֣���1�֣�
��4��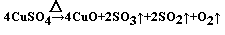 ��2�֣���������ȷ1�֣���ƽ1�֣�
��2�֣���������ȷ1�֣���ƽ1�֣�
���������������1����������������¶Ƚϸ�Ӧѡ������������ͭ���������֬�������ȼ���Һϴȥ��ԭ������֬�ڼ��������·���ˮ�����ȥ�������ա���ͭ������õIJ����ǻ�������ͭ������ͭ����������ͭ�Ŀ���ԭ����a�����չ����в�������ͭ����ԭ d�����ղ����ͭδ����ȫ������
��2���������Ҫ��������Ũ��Ȼ����ȴ�ᾧ������ϴ�ӣ��������Ȼ���;���������Ե������ŵ㣺�ٶ��շ�Ӧת�������֪��������������;��I���������٣���;��I��������Ⱦ���������塣
��3���ⶨ�������������˵��ˮ�����϶�������������٣�a�����Ⱥ�����δ�������������ȴ��һ��������b��������μ��Ⱥ���������ϴ�Ҳ���ܻ�ʹˮ��������С�� c������ǰ����ʱ����δ��ȫ�������ˮ����ʹˮ�������࣬��ȷ��d�����ȹ�������������ʧ������ʹʣ�µĹ����������٣��൱��ˮ�������࣬��ȷ��
��4��������ˮ����ͭ��ĩֱ����ȫ�ֽ⣬A���Թ���ʣ���ɫ��ĩ���ô����ǵ�ľ�����뼯��ƿD������ľ���ܸ�ȼ��˵�����������ɡ��ɼ�¼�����ݿɵó�������������������4.5g������������0.5g�����ɹ�������45g�����ݵ����غ㣬����������0.5g��ʧ������(0.5/32)*4mol���õ��ӿ����������˶����������ɶ����������Ϊ��(0.5/32)*2mol������Ϊ2g��������������Ӧ����������������Ϊ2.5g�����ʵ���Ϊ2.5/80mol,�����ʵ���֮�ȣ���������������������=2��2��1������ԭ���غ��֪���ɺ�ɫ����Ϊ����ͭ����ѧ����ʽΪ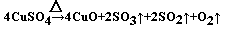 ��
��
���㣺���⿼����ʵ���ԭ����������������


 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ������ȡ�����������װ����ͼ��ʾ�������������������գ�
��1��Բ����ƿ�м���ķ�Ӧ�����廯�ơ� ��1:1�����ᡣ���������1��1���������õĶ�������Ϊ ��ѡ���ţ���
a����ƽ b����Ͳ c������ƿ d���ζ���
��2��д������ʱ��ƿ�з�������Ҫ��Ӧ�Ļ�ѧ����ʽ ��
��3���������ﵼ��ʢ�б�ˮ�������Թ�A�У���ˮ������������ ��
�Թ�A�е����ʷ�Ϊ���㣨��ͼ��ʾ���������ڵ� �㡣
��4���Թ�A�г��˲����ˮ֮�⣬�����ܴ��� ��
(д����ѧʽ)��
��5����Ũ���������ʵ�飬���Թ�A�л�õ��л�����ػ�ɫ����ȥ�������ʵ���ȷ������ ��ѡ���ţ���
a������ b������������Һϴ��
c�������Ȼ�̼��ȡ d��������������Һϴ��
���Թ�B�е����Ը��������Һ��ɫ��ʹ֮��ɫ�����ʵ������� ��
��6��ʵ��Ա��ʦ���������װ���е��������Ӳ��ֶ��ijɱ������ӿڣ���ԭ���ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��������ȼ�ջ����������������Ҫ�ɷ���SO2��CO2��N2��O2 ��ij�о���ѧϰС����ʵ������������װ���Ʊ�ģ��������������ģ������ͨ��ת����������Ч�ʡ�
�ش��������⣺
��ģ���������Ʊ�
��1����Aװ����SO2����ѧ��Ӧ����ʽΪ ��
��2����Bװ����CO2��ʹ�ø�װ���ŵ���� ��
��3�����Ƶõ������������ֻ�ϣ����ģ���������ں���ʵ�顣
II���ⶨ������SO2���������
��4��������ģ����������ͨ��C��Dװ�ã�����C��D��ʢ�е�ҩƷ�ֱ��� �� ��������ţ�
��KMnO4��Һ���ڱ���NaHSO3��Һ���۱���Na2CO3��Һ���ܱ���NaHCO3��Һ
��5����ģ������������ΪamL/min����t1���Ӻ����Ͳ��Һ�����ΪVmL����SO2����������� ��
����ģ������ͨ��ת����������Ч�ʣ���ת����SO2ռԭ��SO2�İٷֱȣ�
��6����ģ������ͨ��ת����Eװ�ã�Eװ����ʢ��FeCl2��FeCl3�Ļ����Һ�������£����ɴ�SO2��O2�ķ�Ӧ���Դﵽ����Ŀ�ġ�д���������뷴Ӧ���̵����ӷ���ʽ ��SO2+2H2O+2Fe3+��SO42-+2Fe2++4H+ �� ��
��7����ģ����������amL/min������ͨ��ת����Eװ�ã�t2���Ӻ���Eװ���м����ữ��BaCl2��Һ�õ���ɫ����Һ���� ��ϴ�ӡ� ���������õ�mg���塣��ʵ����������������Ϊ��״�������ת����������Ч���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���Ȼ�������ɫҺ�壬�е�Ϊ136�档������ˮ�⣬�������е�ˮ���������������̡�(TiCl4��H2O=TiOCl2��2HCl��)����650��850���£�������ͨ���������Ѻ�̿�۵Ļ����ɵõ����Ȼ��Ѻ�һ���ж����塣��ͼ��ij����С���Ʊ�TiCl4�ķ�Ӧװ�ã�����Ҫ�����������£�
�����Ӻ�����װ�ã���ͨCl2ǰ��ͨ��CO2���岢����һ��ʱ�䣻
�ڵ���ƿ��TiCl4������������ʱ��ֹͣ���ȣ��Ӳ���и�ͨCO2����ֱ����¯�еĴɹ���ȴΪֹ��
�۽�TiO2��̿�ۻ�Ͼ��Ⱥ�װ���ʽ��¯�У�
�ܽ���¯���µ�800�棬һ��ʱ����ͨCl2��ͬʱ����������ͨ����ˮ��
�Իش��������⣺
(1)��ȷ�IJ���˳��Ϊ(�����)________��
(2)Cװ���еķ�Ӧ�Ļ�ѧ����ʽΪ_________________________________��
(3)B�е��Լ�Ϊ________����������__________________________��
(4)Dװ�õ�������_________________________________________��
(5)���������ˮCaCl2��������_____________________________��
(6)�ӻ��������ĽǶȣ����һ���Ż�������__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���ڼ���ʱ���뵪����������Ӧ���⻯����ˮ������Ӧ�����������ƺ��������⻯��ͨ��������������Ƽ�����ȡ��ͼ1�Ǻ�����ȡװ�á�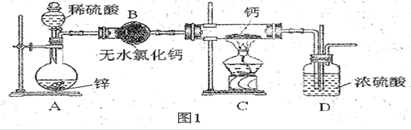
��1����Ũ��������l��4(�����)��ϡ���ᣬ���õIJ����������ձ���________��
��2��װ��D����ֱ���ܵ�������________________________��
��3��Ϊ��ȷ�Ͻ���װ��C�������Ѿ��������B��C֮���ٽ�һװ�ã���װ���м�����Լ���_______������Cװ��ǰҪ��H2�鴿�������ǣ��ռ�һ�Թ����壬���ܿڿ����ƾ��ƻ��棬�����������ۡ���������˵��H2__________________________��
��4����ͬѧ��ΪֻҪװ�ú����������淶�Ϳ����ų�����__________(ѡ�����)��
a��Ca3N2 b��CaO c��Ca(OH)2
��5����ͬѧ����ͼװ�òⶨ�Ƶõ��⻯�ƵĴ��ȡ�����ȡ46 mg��Ʒ��������ˮ��Ӧ������ʱ��ע������������������Ϊ48.06 mL(�ѻ���Ϊ��״��)������ʵ������ԭ�������_______��ѡ���ţ���
��H2ͨ�벻�㣬��Ӧ�����п���
�ڸ���H2δ��ַ�Ӧ
�۲���������Ӵ�
��6����ͬѧ������ͬѧ��ʵ����������һ����ϵʽ��42x+40y=0.046��2x+y=48.06/22400��ָ��ʽ����y�ĺ���___________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�о���ѧϰС��Ϊ�ⶨNH3�����е�����ԭ�Ӹ����ȣ��������ʵ�����̣�
ʵ��ʱ�������Ƶõİ����ž�ϴ��ƿǰ����װ���еĿ�����������ϴ��ƿ�������ռ�װ�ã�������������ͭ��
��ͼA��B��CΪ��С����ȡ����ʱ�����õ���װ�ã�DΪʢ��Ũ�����ϴ��ƿ��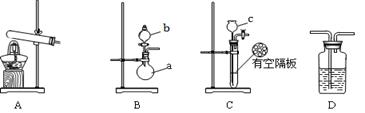
��ش��������⣺
��1��д�����������ƣ�a ��b ��
��2��Ӳ�ʲ������з����ķ�Ӧ����ʽ�� ������Ӧ������Ӳ�ʲ����ܵ������� ��
��3�����ж���ȡ���������õ���װ�ã����±�������Ϊ���е�װ������д��Ӧ��ʵ��ҩƷ��д����ѧʽ����
| װ�� | ʵ��ҩƷ |
| A | |
| B | b�� a�� |
| C | c�� ���壺 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������ʵ���������ÿ�����þ��Ϊԭ����ȡ��������þ(Mg3N2)����֪ʵ���п��ܻᷢ�����з�Ӧ��
��2Mg+O2 2MgO����3Mg+N2
2MgO����3Mg+N2 Mg3N2����2Mg+CO2
Mg3N2����2Mg+CO2 2MgO+C��
2MgO+C��
��Mg+H2O MgO+H2���� ��Mg3N2 +6H2O
MgO+H2���� ��Mg3N2 +6H2O 3Mg(OH)2+2NH3��
3Mg(OH)2+2NH3��
�ɹ�ѡ���װ�ú�ҩƷ����ͼ��ʾ(þ�ۡ���ԭ���۾��Ѹ��װ�����������ķ�Ӧ����ȫ�ģ�����װ�õ�ĩ������������)��
�ش��������⣺
��1�������ʵ�鷽��ʱ����װ��A��D��E�⣬��Ӧѡ���װ���� ������ĸ���ţ���ѡ��װ��DĿ��Ϊ_____________________________ ��
��2��ͨ����Ӧ�ȵ�ȼ ���ľƾ��ƣ����ͬʱ��ȼA��Fװ�õľƾ��ƣ�����ʹʵ���� ���ƫ�ߡ���ƫ�͡���ԭ��
��3�������һ��ʵ�飬��֤������Mg3N2��д���������衢����ͽ��ۣ�
_____________________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�±��е�ʵ������ܴﵽʵ��Ŀ�Ļ��ܵó���Ӧ���۵���
| ѡ�� | ʵ������ | ʵ��Ŀ�Ļ�ʵ����� |
| A | ��ʢ��2 mL 0.1 mol/L AgNO3��Һ���Թ��еμ�5��0.1 mol/L NaCl��Һ���а�ɫ�������ɣ��������еμ�5��0.1 mol/L KI��Һ | ˵��һ�ֳ�����ת��Ϊ�ܽ�ȸ�С�ij��� |
| B | ��1 mL 20% ��������Һ�м���3��5��ϡ���ᣬˮԡ����5 min����ȴ���ټ�������Cu(OH)2����Һ������ | ֤�������ܷ���ˮ�ⷴӦ |
| C | ˮԡ����Ũ���ᡢŨ����ͱ��Ļ�����ֱ�������Һ��õ��Ĵֲ�Ʒ | �Ʊ��������� |
| D | ������,�ֱ���2֧�Թ��м�����ͬ�������ͬŨ�ȵ�Na2S2O3��Һ,�ٷֱ����������ͬŨ�ȵ�ϡ���� | �о�Ũ�ȶԷ�Ӧ���ʵ�Ӱ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ͼ��ʾ��һ��ʵ��������װ�ã����ڷ�����������ռ����塣���и�������������������װ�ý���ʵ�����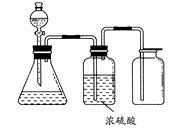
| A��ͭм��ϡ���� |
| B���������̺�Ũ���� |
| C����Ũ��ˮ����ʯ�ҷ�Ӧ |
| D��̼��ƺ�ϡ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com