
�ײ��к��и�����Ԫ�أ�ij��ѧС��������·����ⶨ�ɰײ��и�Ԫ�ص���������������ȡ10.00g�ɰײ�Ҷ�����յðײ˻ҷ۽�������ʵ�飺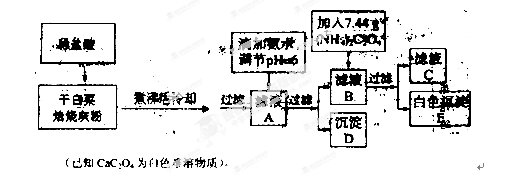
��1��ʵ��ǰҪ�Ƚ��ɰײ�Ҷ��Ʒ�������ճɻҷۣ�����ҪĿ����ʹ��Ʒ�е��л�����ȫ�ֽ⣬ʹ�ɰײ�Ҷ�еĸơ���Ԫ���ܽ���ȫ�������õ��IJ���������
A������ B�������� C�������� D��������
��2��д������ҺA������D�����ӷ�Ӧ����ʽ ��
��3����KMnO4����Һ�ζ���ҺC���Ƚ���ҺCϡ����500 mL����ȡ���е�25��00 mL��Һ���������ữ����0.100 0 mol��L-1����KMnO4����Һ�ζ����յ�ʱ����KMnO4��Һ10.00mL��
�����ķ�ӦΪ��
�ٵζ��Ĺ����У�ͬѧ�Ƿ���һ��������C��Һ�м����һ��KMnO4��Һʱ����Ҫ��ҡ��ƿ�ϳ�ʱ�������ɫ������Һ��ɫ���ٵ���KMnO4��Һ����Ѹ����ɫ��ֱ���ﵽ�յ㣻Ϊ�˼ӿ�����һ��KMnO4��Һʱ����ɫ�ٶȣ��ɲ�ȡ�ķ����� .��ѡ����ʵ�ѡ�
A���ʵ�������ƿ����Һ B������ƿ�ڼ�����ˮ
C������ƿ�ڼ������Ҵ� D������ƿ�ڼ��뼸��MnSO4��Һ
���жϵζ��ﵽ�յ�ķ����� ��
��4��ԭ�ɰײ�Ҷ�и�Ԫ�ص���������Ϊ ��
��5��Ϊ��֤ʵ�龫ȷ�ȣ�����D��E��Ҫ�ֱ�ϴ�ӣ�����ϴ��Һת�ƻ�ĸҺ�У����жϳ���D�Ѿ�ϴ�Ӹɾ��ķ����� ���������Eδϴ�ӣ���δ��ϴ��Һת�ƻ�ĸҺ�����õĸ�Ԫ���������� ���ƫ�ߡ���ƫ�͡�����Ӱ�족����
����13�֣���ѧ��Ӧʽ��ʽδ��ƽ�ľ���1��
��1�� ACD��2�֣���ѡ1����1�֣��д�ѡ���÷֣�
��2�� Fe3����3NH3��H2O��Fe(OH)3����3NH4+��2�֣���NH3��H2O��д�ɡ�NH3��H2O�����۷֣��ޡ�������1�֣�д�ɻ�ѧ����ʽ���÷֣�
��3���� AD ��2�֣���ѡ1����1�֣��д�ѡ���÷֣�
�� �������һ��KMnO4��Һ����Һ�Ժ�ɫ�Ұ��������Һ����ɫ����1�֣��ᵽ����Һ����ɫ������ɫ�����Ϻ�ɫ����ɫ�����ɵ÷֣�
��4��4.000%��3�֣���Ч���ֲ����ǣ�
��5��ȡ���һ��ϴ��Һ�������Թ��У��μ�Na2CO3��Һ������������������ϴ�Ӹɾ�����2�֣��Լ�1�֣�����1�֣�������������Ҳ���֣�
ƫ�ߣ�1�֣�
�����������������ҪŪ���������Ԫ�����������ⶨ�Ĺ������̡����ײ˾���һϵ�еIJ������õ���ҺA����ҺA�к��е�������Fe3����Ca2+�����백ˮ����pH=6��Ŀ���ǽ�Fe3����ȥ��Ȼ��õ���ҺB�ͳ���D Fe(OH)3������ҺB�м������泥�Ŀ���ǽ�Ca2+������ɲ���ƣ�����ɫ����E��ͨ���ⶨ��ɫ����E���������ⶨ�ײ��и�Ԫ�ص�����������
���ոɰײ�Ҷ����Ҫ��������Ҫ�У����������������ƾ��ƣ������ǡ��������ᾧ��������������
����ҺA������D�����ӷ�Ӧ����ʽΪ��Fe3����3NH3��H2O��Fe(OH)3����3NH4+
��ҺC���й����IJ���泥�ͨ���ø��������Һ���ⶨ�����IJ���泥��Ӷ����Եó���Ca2+��Ӧ�IJ���泥��Ӷ�����������ӵĺ�����
��Ϊ�˼ӿ�����һ��KMnO4��Һʱ����ɫ�ٶȣ����ӿ췴Ӧ�ٶȣ������ʵ��ļ�����ƿ�ڵ���Һ��Ҳ���Լ��뼸��MnSO4��Һ����ΪMn2+�д��÷�Ӧ�ٶȵ����á�
�ڵ���Ӧ�ﵽ�ζ��յ�ʱ��Ҳ�������һ�θ��������Һ���룬������Ѿ��������ˣ���ʱ��Һ����ɫ�����ɫ������30s�ڲ���ɫ�����ζ��ﵽ�յ㡣
ԭ�ɰײ�Ҷ�и�Ԫ�ص����������IJⶨ��
��Һ�й�����n��C2O42-��=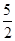 n��MnO4-��=
n��MnO4-��=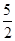 ��0.1��0.01="0.0025" mol
��0.1��0.01="0.0025" mol
����Ca2+��Ӧ�IJ����n��C2O42-��=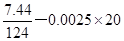 =0.01mol
=0.01mol
����n��Ca2+��= n��C2O42-��=0.01mol
w��Ca��= =4.000%
=4.000%
��5���жϳ����Ƿ�ϴ������Ҫ���������Ƿ��п����Ե����ӣ�����ȡ���һ��ϴ�ӵ���Һ�������Һ���Ƿ���Ca2+ ���ɡ����������ȡ���һ��ϴ��Һ�������Թ��У��μ�Na2CO3��Һ������������������ϴ�Ӹɾ�������EΪ����ƣ������Ͽ��ܸ��п����������磺C2O42-����δϴ�ӣ�������ø��������Һ�ζ���Һ�е�C2O42-�������ĵĸ�����ص���ƫ�٣���ô������ӷ�Ӧ�IJ�������ӵ�����ƫ�ߣ����ղ�õİײ�Ҷ�еĸ����ӵĺ���ƫ�ߡ�
���㣺����Ԫ�صĶ�������ʵ�飬����ʵ��������̡�����Ҫ��������ʵ�鶨��������ԭ����


 ��Ȥ����¹�֪��ϵ�д�
��Ȥ����¹�֪��ϵ�д� Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д� �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��������Ҫ�ɷ�ΪFeS2�����ҹ���������᳧��ȡ�������Ҫԭ�ϡ�ij��ѧѧϰС���ij������ʯ��������ʵ��̽����
[ʵ��һ]�ⶨ��Ԫ�صĺ�����
��m1 g�û�������Ʒ��������ͼ��ʾװ�ã��гֺͼ���װ��ʡ�ԣ���ʯӢ���У���a�����ϵػ���ͨ���������������ʯӢ���еĻ�������Ʒ����Ӧ��ȫ��ʯӢ���з�����Ӧ�Ļ�ѧ����ʽΪ��4FeS2+11O2 2Fe2O3+8SO2
2Fe2O3+8SO2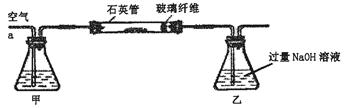
��Ӧ��������ƿ�е���Һ�������´�����
|

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�������ƣ�CaO2��������������������ҩ�����졢��֬Ư���������������� ������Ϊ������������������ף��������Ƶ��Ʊ�����һ�������ַ���������CaCl2�ڼ�����������H2O2��Ӧ��������Ca(OH)2��NH4Cl��Һ��H2O2��Ӧ�����ɵõ�CaO2��8H2O��������֪CaO2��8H2O�ʰ�ɫ������ˮ����60���º�0.5Сʱ���γ�CaO2��2H2O������ 140���º�0.5Сʱ������ˮCaO2��������350�����ҿ�ʼ�ֽ�ų�������
��֪ij�����������������Ƶ���Ҫ�����������£�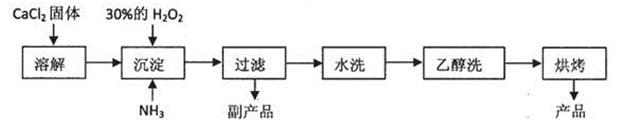
ij��ѧʵ��С����ʵ������ģ�����������Ƶò�Ʒ���ⶨ����CaO2�ĺ�����
��1��������������ȡCaO2��8H2O�Ļ�ѧ����ʽ�ǣ� ��
��2�����顰ˮϴ���Ƿ�ϸ�ķ����ǣ� ��
��3������ʱ���ñ�ˮ�����¶���0�����ң�����ܵ�ԭ�������
�ٸ÷�Ӧ�Ƿ��ȷ�Ӧ���¶ȵ����������CaO2��8H2O���ʣ�
�� ��
��4���ⶨ��Ʒ��CaO2�ĺ�����ʵ�鲽���ǣ�
��һ����ȷ��ȡa g��Ʒ��������ƿ�У�������������ˮ������b g KI���壬 �ٵ�������2mol/L��������Һ����ַ�Ӧ��
�ڶ�������������ƿ�м��뼸�ε�����Һ��
����������μ���Ũ��Ϊc mol/L��Na2S2O3��Һ����Ӧ��ȫ���ζ����յ㣬��¼���ݣ����ظ������ⶨ����һ�����������Σ��ó�����ƽ������Na2S2O3��Һ����ΪVmL
����֪��I2+2S2O32��=2I��+ S4O62����
�������������ζ����յ㣬����Ϊ ��
��CaO2����������Ϊ ������ĸ��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����ˮ�м���������ȩ��Һ,���Կ�����ˮ��ɫ���ݴ˶���ˮ����ȩ�������л���Ӧ���ͽ�������̽�����������������գ�
I���²⣺
��1����ˮ����ȩ����ȡ����Ӧ��
��2����ˮ����ȩ�����ӳɷ�Ӧ��
��3����ˮ����ȩ����_______��Ӧ��
II����Ʒ�������֤��
Ϊ̽����һ�ֲ²���ȷ,ij�о���ѧϰС���������������ʵ�鷽����
����l��������ɫ����Һ������ԡ�
����2���ⶨ��Ӧǰ������ˮ�Ʊ���Br2�����ʵ����ͷ�Ӧ��Br-���ӵ����ʵ�����
��1������1�Ƿ���У� _______��������__________________________________________
��2�������÷�Ӧǰ������ˮ�Ʊ���Br2�����ʵ���Ϊa mol ��
����÷�Ӧ��n(Br��) = _______mol����˵����ˮ����ȩ�����ӳɷ�Ӧ����
����÷�Ӧ��n(Br-) = _______mol����˵����ˮ����ȩ����ȡ����Ӧ��
����÷�Ӧ��n(Br-) = _______mol����˵���²�(3)��ȷ��
III��ʵ����֤:ijͬѧ�ں�0.005molBr2��10mL��Һ�У�����������ȩ��Һʹ����ɫ���ټ������AgNO3��Һ���õ�����ɫ����l.88 g����֪��Ӧ�����л�����AgNO3����Ӧ�������ݼ���������֪��ˮ����ȩ��Ӧ�����ӷ���ʽΪ__________________________________________��
IV����չ
������ƶ���ʵ��,̽����ȩ���Ҵ��Ļ�ԭ��ǿ��(��д�±�����
| ʵ��������� | ʵ������ | ���� |
| | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
CuSO4��5H2O��ͭ����Ҫ��������Ź㷺��Ӧ�á�ʵ�����ô�ͭ�������������Ʊ�CuSO4��5H2O���������£�
�ش��������⣺
��1��ʵ��������250 mL4.8 mol��L��1��ϡ���ᣬ����IJ�������������������Ͳ���ձ������Ҫ__________________________________________________��
��2����֪��ͬ����������������������������pH���±���
| ���� | Fe3+ | Cu2+ | Fe2+ |
| ��ʼ������pH | 2.7 | 4.4 | 7.0 |
| ������ȫ��pH | 3.7 | 6.4 | 9.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����ʵ�鷽������У����е���( )
| A����ϡ�������ˣ���ȥ����ͭ���е�����þ�ۺ����� |
| B������ȡ�ķ����������ͺ�ú�� |
| C�����ܽ⡢���˵ķ�����������غ��Ȼ��ƹ���Ļ���� |
| D���������������Ļ������ͨ�����ȵ�����ͭ���Գ�ȥ���е����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����ʵ�鲻�ܴﵽĿ�ĵ���
| A���ñ���NaHCO3��Һ��ȥCO2�л��е�HCl |
| B���ü�������CuCl2��Һ�ķ����Ʊ���ˮCuCl2���� |
| C���÷�Һ©������CCl4��ȡ��ˮ���ѷֲ���л����ˮ�� |
| D���ü��ȷֽ�ķ�������̼���ƺ�̼���������ֹ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���Т�������������������Һ�����廯����Һ����ˮ���۱����屽�Ļ��Һ���������ǵ���ȷ����������
| A����Һ������Һ | B����ȡ����Һ������ |
| C����Һ����ȡ������ | D������Һ����ȡ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com