
(12��) ijһ��Ӧ��ϵ�У��з�Ӧ��������ﹲ�������ʣ������������ǣ�Cl2��KMnO4��MnCl2��H2O��HCl(Ũ)��KCl������KMnO4 �� MnCl2 ��
(1)�÷�Ӧ�еĻ�ѧ����ʽΪ_____________________________________________��
(2)�÷�Ӧ�У����������뻹ԭ��������ʵ���֮��Ϊ_____________________________��
(3)�������������ڱ�״�������Ϊ2.24 L����Ӧ�����б�����������Ϊ________ NA��NA��ʾ����٤��������ֵ����
(4)���۵�Ũ���ᣨ�ܶ�Ϊ1.19g/cm3���ڹ�ҵ������500 L HCl����(��״��)��1 L H2O�ı������Ƴɵģ������������ʵ���Ũ����___________mol/L(�������һλС��)��
(5)������1.20 mol/L��ϡ����480 mL, Ӧ��ȡ����Ũ����________mL�������ơ�
(5)ȡ6.00 gӲ���Ͻ𣨼���ֻ����ͭ�裩���� (5) ������ϡ������г�ַ�Ӧ���ռ�������5.60 L(��״��)����Ӳ���Ͻ���������������Ϊ____________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12��) ijһ��Ӧ��ϵ�У��з�Ӧ��������ﹲ�������ʣ������������ǣ�Cl2��KMnO4��MnCl2��H2O��HCl(Ũ)��KCl������KMnO4 �� MnCl2 ��
(1)�÷�Ӧ�еĻ�ѧ����ʽΪ_____________________________________________��
(2)�÷�Ӧ�У����������뻹ԭ��������ʵ���֮��Ϊ_____________________________��
(3)�������������ڱ�״�������Ϊ2.24 L����Ӧ�����б�����������Ϊ________ NA��NA��ʾ����٤��������ֵ����
(4)���۵�Ũ���ᣨ�ܶ�Ϊ1.19g/cm3���ڹ�ҵ������500 L HCl����(��״��)��1 L H2O�ı������Ƴɵģ������������ʵ���Ũ����___________mol/L(�������һλС��)��
(5)������1.20 mol/L��ϡ����480 mL, Ӧ��ȡ����Ũ����________mL�������ơ�
(5)ȡ6.00 gӲ���Ͻ𣨼���ֻ����ͭ�裩����(5) ������ϡ������г�ַ�Ӧ���ռ�������5.60 L(��״��)����Ӳ���Ͻ���������������Ϊ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����������ϴ�ѧ������ѧ��һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
(12��) ijһ��Ӧ��ϵ�У��з�Ӧ��������ﹲ�������ʣ������������ǣ�Cl2��KMnO4��MnCl2��H2O��HCl(Ũ)��KCl������KMnO4 �� MnCl2��
(1)�÷�Ӧ�еĻ�ѧ����ʽΪ_____________________________________________��
(2)�÷�Ӧ�У����������뻹ԭ��������ʵ���֮��Ϊ_____________________________��
(3)�������������ڱ�״�������Ϊ2.24 L����Ӧ�����б�����������Ϊ________ NA��NA��ʾ����٤��������ֵ����
(4)���۵�Ũ���ᣨ�ܶ�Ϊ1.19g/cm3���ڹ�ҵ������500 L HCl����(��״��)��1 L H2O�ı������Ƴɵģ������������ʵ���Ũ����___________mol/L(�������һλС��)��
(5)������1.20 mol/L��ϡ����480  mL, Ӧ��ȡ����Ũ����________mL�������ơ�
mL, Ӧ��ȡ����Ũ����________mL�������ơ�
(5)ȡ6.00 gӲ���Ͻ𣨼���ֻ����ͭ�裩���� (5) ������ϡ������г�ַ�Ӧ���ռ�������5.60 L(��״��)����Ӳ���Ͻ���������������Ϊ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012����У�Ϻ��У�����������ʦ������У�����������ѧ�Ծ����������� ���ͣ�ʵ����
�����12�֣�
ij��ѧ����С�����������ͼ��ʾ��װ�ý���ʵ�顣ͼ�м�ͷ��ʾ��������A��ʾһ�ִ�������������壬B����һ�����壬��Ӧ����һ��ʱ���װ�ü����к���ɫ�������ɡ�ʵ�������õ�ҩƷ�����ֻ�ܴ�����������ѡȡ��Na2CO3��NaHCO3��MnO2��Na2O2��NaCl����ˮCaCl2 ��NH4HCO3����ʯ�ҵȹ��������ˮ��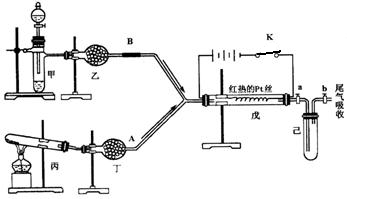
����ͼ��װ�úͷ�Ӧ������ش�
��1�����з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2�����еĸ����Ӧѡ ________����ѡ��һ�ָ�������� ��
��3�����з�����Ӧ�Ļ�ѧ����ʽΪ ��
��4�����з�������Ҫ��Ӧ�Ļ�ѧ����ʽΪ____________________���˷�Ӧ��________�����ȡ����ȣ���Ӧ����֤������жϵ������� ��
��5�������г�������ɫ�����ֹͣ�������ȣ����ر�a��b���������������������ˮ�У������л���ֵ������ǣ�______________�������������ԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ֣���и�����һ������Ԥ�⻯ѧ�Ծ��������棩 ���ͣ�ʵ����
(12�֡�ij�о���ѧϰС����̽��SO2�ܷ���BaCl2��Һ��Ӧ����BaSO3�������������ϵ�֪������BaSO3��KSPΪ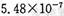 ��������������
��������������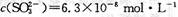 ��
��
(1) ��0.1 mol ? L��1��BaCl2��Һ���뱥���������У�_______ (��ܡ����ܡ�������BaSO3������ԭ����______________ (��д����Ҫ���ƶϹ��̣���
(2) Ũ����ķе�Ϊ338��C���ƾ��ƻ�����¶�Ϊ400?5000C����ͬѧ��װ��I����ʵ�飬����BaCl2��Һ�г��ְ�ɫ�������Ұ�ɫ�������������ᡣ
��д�������Թ��з�����Ӧ�Ļ�ѧ����ʽ��_____________________
�ڰ�ɫ�����Ļ�ѧʽ��_______���������ӷ���ʽ��ʾ���ɸð�ɫ�����Ŀ���ԭ��___________________________________
(3) ��ͬѧ��Ϊ��ͬѧ��װ�ò����ƣ�����˸Ľ�װ��II����ʵ�飨�г�װ�ú�A�м���װ�����ԣ��������Ѽ��飩��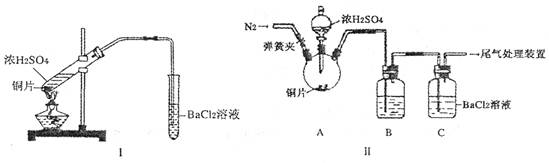
�ٴ��ɼУ�ͨ��N2����ʱ���رյ��ɼ�
�ڵμ�һ����Ũ���ᣬ����A��һ��ʱ���C��δ���������ɡ�
�����ٵ�Ŀ����_______��ϴ��ƿB�е��Լ���______________��
(4) ��ͬѧȡ��ʵ����C����Һ�������μ�һ����ɫ��Һ��Ҳ��������������İ�ɫ���������μӵ��Լ�������______________��
| A��NaOH��Һ | B��Na[Al(OH)4]��Һ | C��H2O2��Һ | D������ KMnO4��Һ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com