
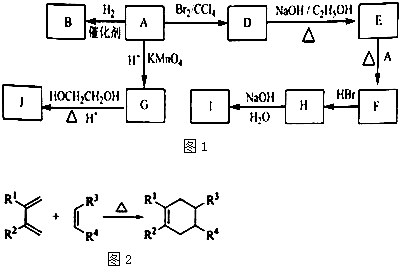
(12·Ö)ÓŠ»ś»ÆŗĻĪļA”«GÓŠČēĻĀ×Ŗ»Æ¹ŲĻµ£ŗ

ŅŃÖŖ£ŗ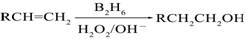 £ØB2H6ĪŖŅŅÅšĶ飩
£ØB2H6ĪŖŅŅÅšĶ飩
ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
£Ø1£©11.2L£Ø±ź×¼×“æö£©µÄĢžAŌŚŃõĘųÖŠ³ä·ÖČ¼ÉÕæÉŅŌ²śÉś88g CO2ŗĶ45g H2O ”£AµÄ·Ö×ÓŹ½ŹĒ£ŗ ”£
£Ø2£©BŗĶC ¾łĪŖŅ»ĀČ“śĢž£¬ĖüĆĒµÄ¹ŲĻµŹĒ ”£
£Ø3£©·“Ó¦¢ŁµÄ·“Ó¦ĄąŠĶŹĒ£ŗ ”£
£Ø4£©·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½ŹĒ£ŗ
ӣ
£Ø5£©ŗ¬ÓŠĻąĶ¬¹ŁÄÜĶŵÄFµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹Ź½ŹĒ£ŗ
ӣ
£Ø6£©ŌŚŅ»¶ØĢõ¼žĻĀ£¬ÓÉD¾ŪŗĻµĆµ½µÄøß·Ö×Ó»ÆŗĻĪļµÄ½į¹¹¼ņŹ½ŹĒ£ŗ
ӣ
 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(12·Ö)ÓŠ»ś»ÆŗĻĪļA”«GÓŠČēĻĀ×Ŗ»Æ¹ŲĻµ£ŗ
ŅŃÖŖ£ŗ £ØB2H6ĪŖŅŅÅšĶ飩
ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
£Ø1£©11.2L£Ø±ź×¼×“æö£©µÄĢžAŌŚŃõĘųÖŠ³ä·ÖČ¼ÉÕæÉŅŌ²śÉś88g CO2ŗĶ45g H2O”£AµÄ·Ö×ÓŹ½ŹĒ£ŗ ”£
£Ø2£©BŗĶC ¾łĪŖŅ»ĀČ“śĢž£¬ĖüĆĒµÄ¹ŲĻµŹĒ ”£
£Ø3£©·“Ó¦¢ŁµÄ·“Ó¦ĄąŠĶŹĒ£ŗ ”£
£Ø4£©·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½ŹĒ£ŗ
ӣ
£Ø5£©ŗ¬ÓŠĻąĶ¬¹ŁÄÜĶŵÄFµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹Ź½ŹĒ£ŗ
ӣ
£Ø6£©ŌŚŅ»¶ØĢõ¼žĻĀ£¬ÓÉD¾ŪŗĻµĆµ½µÄøß·Ö×Ó»ÆŗĻĪļµÄ½į¹¹¼ņŹ½ŹĒ£ŗ
ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010ÄźĮÉÄžŹ”±¾ĻŖŹŠøßŅ»ĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧA¾ķ ĢāŠĶ£ŗĢīæÕĢā
(12·Ö)ÓŠ»ś»ÆŗĻĪļA”«GÓŠČēĻĀ×Ŗ»Æ¹ŲĻµ£ŗ
ŅŃÖŖ£ŗ £ØB2H6ĪŖŅŅÅšĶ飩
£ØB2H6ĪŖŅŅÅšĶ飩
ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
£Ø1£©11.2L£Ø±ź×¼×“æö£©µÄĢžAŌŚŃõĘųÖŠ³ä·ÖČ¼ÉÕæÉŅŌ²śÉś88g CO2ŗĶ45g H2O”£AµÄ·Ö×ÓŹ½ŹĒ£ŗ ”£
£Ø2£©BŗĶC¾łĪŖŅ»ĀČ“śĢž£¬ĖüĆĒµÄ¹ŲĻµŹĒ ”£
£Ø3£©·“Ó¦¢ŁµÄ·“Ó¦ĄąŠĶŹĒ£ŗ ”£
£Ø4£©·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½ŹĒ£ŗ
ӣ
£Ø5£©ŗ¬ÓŠĻąĶ¬¹ŁÄÜĶŵÄFµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹Ź½ŹĒ£ŗ
ӣ
£Ø6£©ŌŚŅ»¶ØĢõ¼žĻĀ£¬ÓÉD¾ŪŗĻµĆµ½µÄøß·Ö×Ó»ÆŗĻĪļµÄ½į¹¹¼ņŹ½ŹĒ£ŗ
ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģø£½ØŹ”ø߶žÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
( 12·Ö)ÓŠĘßÖÖŌŖĖŲ£¬ĘäÖŠA”¢B”¢C”¢D”¢EĪŖ¶ĢÖÜĘŚÖ÷×åŌŖĖŲ£¬F”¢GĪŖµŚĖÄÖÜĘŚŌŖĖŲ£¬ĖüĆĒµÄŌ×ÓŠņŹżŅĄ“ĪŌö“ó”£Ēė»Ų“šĪŹĢā”£
|
AŌŖĖŲµÄŗĖĶāµē×ÓŹżŗĶµē×Ó²ćŹżĻąµČ£¬Ņ²ŹĒÓīÖęÖŠ×ī·įø»µÄŌŖĖŲ |
|
BŌŖĖŲŌ×ÓµÄŗĖĶāpµē×ÓŹż±Čsµē×ÓŹżÉŁ1 |
|
CŌ×ӵĵŚŅ»ÖĮµŚĖĵēĄėÄÜ·Ö±šŹĒ: I1=738kJ/mol I2 = 1451 kJ/mol I3 = 7733kJ/mol I4 = 10540kJ/mol |
|
DŌ×ÓŗĖĶāĖłÓŠp¹ģµĄČ«Āś»ņ°ėĀś |
|
EŌŖĖŲµÄÖ÷×åŠņŹżÓėÖÜĘŚŹżµÄ²īĪŖ4 |
|
FŹĒĒ°ĖÄÖÜĘŚÖŠµēøŗŠŌ×īŠ”µÄŌŖĖŲ |
|
GŌŚÖÜĘŚ±ķµÄµŚ°ĖĮŠ |
£Ø1£©ŅŃÖŖBA5 ĪŖĄė×Ó»ÆŗĻĪļ£¬ŹĒÓÉ ”¢ Į½ÖÖĪ¢Į£¹¹³ÉµÄ£ØĢī»Æѧ·ūŗÅ£©”£
£Ø2£©B»łĢ¬Ō×ÓÖŠÄÜĮæ×īøߵĵē×Ó£¬Ęäµē×ÓŌĘŌŚæÕ¼äÓŠ øö·½Ļņ£¬Ō×Ó¹ģµĄ³Ź ŠĪ”£
£Ø3£©Ä³Ķ¬Ń§øł¾ŻÉĻŹöŠÅĻ¢£¬ĶʶĻC»łĢ¬Ō×ÓµÄŗĖĶāµē×ÓÅŲ¼ĪŖ£¬
øĆĶ¬Ń§Ėł»µÄµē×ÓÅŲ¼Ķ¼Ī„±³ĮĖ ”£
£Ø4£©GĪ»ÓŚ
×壬G3+¼Ūµē×ÓÅŲ¼Ź½ĪŖ
”£GE3³£ĪĀĻĀĪŖ¹ĢĢ壬ČŪµć £¬·Šµć
£¬·Šµć £¬ŌŚ
£¬ŌŚ ŅŌÉĻÉż»Ŗ£¬Ņ×ČÜÓŚĖ®£¬Ņ²Ņ×ČÜÓŚŅŅĆŃ”¢±ūĶŖµČÓŠ»śČܼĮ”£¾Ż“ĖÅŠ¶ĻGE3µÄ¾§ĢåĄąŠĶĪŖ________________”£
ŅŌÉĻÉż»Ŗ£¬Ņ×ČÜÓŚĖ®£¬Ņ²Ņ×ČÜÓŚŅŅĆŃ”¢±ūĶŖµČÓŠ»śČܼĮ”£¾Ż“ĖÅŠ¶ĻGE3µÄ¾§ĢåĄąŠĶĪŖ________________”£
£Ø5£©DE3 ÖŠŠÄŌ×ÓµÄŌӻƷ½Ź½ĪŖ £¬Ęäæռ乹ŠĶĪŖ ”£
£Ø6£©Ē°ĖÄÖÜĘŚÖŠÓėFĶ¬Ņ»×åµÄĖłÓŠŌŖĖŲ·Ö±šÓėEŌŖĖŲŠĪ³É»ÆŗĻĪļ£¬Ę侧ĢåµÄČŪµćÓÉøßµ½µĶµÄÅÅĮŠĖ³ŠņĪŖ£ØŠ“»ÆѧŹ½£© £¬ŌŅņŹĒ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com