
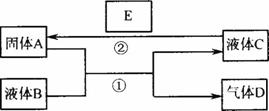
ͼ6-5
(1)��A��һ���Ϻ�ɫ����������D��ʹƷ����Һ��ɫ������ʱ�ֿ��Իָ�ԭɫ��д����Ӧ�ٵĻ�ѧ����ʽ��______________________������D����һ������H��Ϻ�����һ�ֵ���ɫ����W��д���÷�Ӧ�Ļ�ѧ����ʽ��______________________��
(2)��A��һ�ֽ������ʣ�D����������壬B�ܷ���NaOH��Һ?___________(��ܡ���)
(3)��A�ǽ������ʣ�D��һ����ɫ���壬����������Ϊ����ɫ��Һ��C����ɫ��
д����Ӧ�ٵ����ӷ���ʽ��____________________________________________��
д����Ӧ�ڵ����ӷ���ʽ��____________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2007?�Ͳ���ģ����ѧ��ѧ���кܶ����ʿ���ʵ����ͼ������֮���ת�������з�Ӧ�����Ͳ��ַ�Ӧ�IJ�������ȥ��Һ��B��C�����ǵ�һ���ʵ���Һ��Ҳ�����Ǵ����
��2007?�Ͳ���ģ����ѧ��ѧ���кܶ����ʿ���ʵ����ͼ������֮���ת�������з�Ӧ�����Ͳ��ַ�Ӧ�IJ�������ȥ��Һ��B��C�����ǵ�һ���ʵ���Һ��Ҳ�����Ǵ����
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��1����A��һ���Ϻ�ɫ����������D��ʹƷ����Һ��ɫ������ʱ�ָֻ�ԭɫ��д����Ӧ�ٵĻ�ѧ����ʽ��_________________________������D����һ������H��Ϻ�����һ�ֵ���ɫ����W��д���÷�Ӧ�Ļ�ѧ����ʽ��____________________________��������������ͻ�ԭ�������ʵ���֮��Ϊ______________________��
��2����A��һ�ֽ������ʣ�D����������壬B�ܷ���NaOH��Һ_____________����ܡ�����
��3����A�ǽ������ʣ�D��һ����ɫ���壬����������Ϊ����ɫ��Һ��C����ɫ��д����Ӧ�ٵ����ӷ���ʽ��__________________________________��д����Ӧ�ڵ�����һ�����ӷ���ʽ��__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
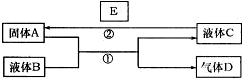
��10�֣���ѧ��ѧ���кܶ����ʿ���ʵ����ͼ������֮���ת�������з�Ӧ�����Ͳ��ַ�Ӧ�IJ�������ȥ��Һ��B��C�����ǵ�һ���ʵ���Һ��Ҳ�����Ǵ����
��1����A��һ���Ϻ�ɫ����������D��ʹƷ����Һ��ɫ������ʱ�ָֻ�ԭɫ��д��A��B��Ӧ�Ļ�ѧ����ʽ ��
��2����A�ǽ������ʣ�D��һ����ɫ���壬����������Ϊ����ɫ��Һ��C����ɫ����A��B��Һ��Ӧ�����ӷ���ʽ ��
д������ɫ������ˮ��Ӧ�Ļ�ѧ����ʽ ��
��3����A�Ǻ�ɫ���嵥�ʣ�D����������Ļ���������һ��������ʹ����ʯ��ˮ����ǣ���A��B��Һ��Ӧ�Ļ�ѧ����ʽΪ ��
��4���������D����ҺB���ܱ�ʪ��ĺ�ɫʯ����ֽ��������÷�Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ���ݰ��и�һ��ѧ�����п��Ի�ѧ�Ծ� ���������� ���ͣ������
��10�֣���ѧ��ѧ���кܶ����ʿ���ʵ����ͼ������֮���ת�������з�Ӧ�����Ͳ��ַ�Ӧ�IJ�������ȥ��Һ��B��C�����ǵ�һ���ʵ���Һ��Ҳ�����Ǵ����
��1����A��һ���Ϻ�ɫ����������D��ʹƷ����Һ��ɫ������ʱ�ָֻ�ԭɫ��д��A��B��Ӧ�Ļ�ѧ����ʽ ��
��2����A�ǽ������ʣ�D��һ����ɫ���壬����������Ϊ����ɫ��Һ��C����ɫ����A��B��Һ��Ӧ�����ӷ���ʽ ��
д������ɫ������ˮ��Ӧ�Ļ�ѧ����ʽ ��
��3����A�Ǻ�ɫ���嵥�ʣ�D����������Ļ���������һ��������ʹ����ʯ��ˮ����ǣ���A��B��Һ��Ӧ�Ļ�ѧ����ʽΪ ��
��4���������D����ҺB���ܱ�ʪ��ĺ�ɫʯ����ֽ��������÷�Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�츣��ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��10�֣���ѧ��ѧ���кܶ����ʿ���ʵ����ͼ������֮���ת�������з�Ӧ�����Ͳ��ַ�Ӧ�IJ�������ȥ��Һ��B��C�����ǵ�һ���ʵ���Һ��Ҳ�����Ǵ����
��1����A��һ���Ϻ�ɫ����������D��ʹƷ����Һ��ɫ������ʱ�ָֻ�ԭɫ��д��A��B��Ӧ�Ļ�ѧ����ʽ ��
��2����A�ǽ������ʣ�D��һ����ɫ���壬����������Ϊ����ɫ��Һ��C����ɫ����A��B��Һ��Ӧ�����ӷ���ʽ ��
д������ɫ������ˮ��Ӧ�Ļ�ѧ����ʽ ��
��3����A�Ǻ�ɫ���嵥�ʣ�D����������Ļ���������һ��������ʹ����ʯ��ˮ����ǣ���A��B��Һ��Ӧ�Ļ�ѧ����ʽΪ ��
��4���������D����ҺB���ܱ�ʪ��ĺ�ɫʯ����ֽ��������÷�Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com