

 N2O4(g) ��H ����52.7kJ��mol-1
N2O4(g) ��H ����52.7kJ��mol-1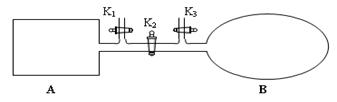
 N2O4�Ѿ��ﵽƽ�⡣
N2O4�Ѿ��ﵽƽ�⡣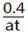 mol��L-1��s-1�ܱ�С
mol��L-1��s-1�ܱ�С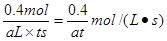 ��
��

 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
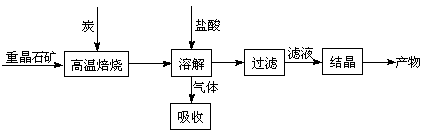
 4CO(g) + BaS(s) ��H1 ����571.2 kJ��mol-1 ��
4CO(g) + BaS(s) ��H1 ����571.2 kJ��mol-1 �� 2CO2(g) + BaS(s) ��H2����226.2 kJ��mol-1 ��
2CO2(g) + BaS(s) ��H2����226.2 kJ��mol-1 �� �� ��[Ksp(AgBr)��5.4��10-13��Ksp(AgCl)��2.0��10-10]
�� ��[Ksp(AgBr)��5.4��10-13��Ksp(AgCl)��2.0��10-10] 2CO(g)�ġ�H�� kJ��mol-1��
2CO(g)�ġ�H�� kJ��mol-1���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2CH3OH(g) ��H= 37Kj��mol-1
2CH3OH(g) ��H= 37Kj��mol-1 3 H2(g)+CO2(g) ��H =49Kj��mol-1
3 H2(g)+CO2(g) ��H =49Kj��mol-1 CO(g) +H2O(g) ��H=41.3Kj��mol-1
CO(g) +H2O(g) ��H=41.3Kj��mol-1 CH3OH(g) ��H <0���ֽ�l0mol CO��20mol H2�����ܱ������У��ڴ��������·�����Ӧ���ɼ״���CO��ƽ��ת���ʣ�
CH3OH(g) ��H <0���ֽ�l0mol CO��20mol H2�����ܱ������У��ڴ��������·�����Ӧ���ɼ״���CO��ƽ��ת���ʣ�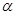 �����¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
�����¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��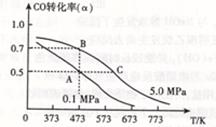
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
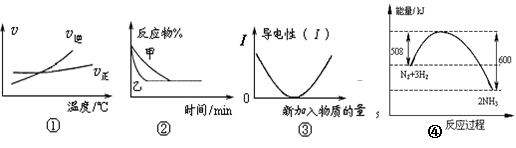
A������ͼ�ٿ��жϿ��淴Ӧ �� A2��g��+3B2��g�� 2AB3��g���� �Ħ�H>0 2AB3��g���� �Ħ�H>0 |
B��ͼ�ڱ�ʾѹǿ�Կ��淴Ӧ2A��g��+ 2B��g�� 3C��g��+ D��s����Ӱ�죬�ҵ�ѹǿ�� 3C��g��+ D��s����Ӱ�죬�ҵ�ѹǿ�� |
| C��ͼ�ۿɱ�ʾ������Һ��ͨ�백����������������Һ�����Եı仯 |
| D��ͼ����N2��H2�ϳɰ��������仯���ߣ���ȷ���÷�Ӧ1 mol N2��3 mol H2��ַ�Ӧʱ����һ��С��92 kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 H2��I2(g)
H2��I2(g) 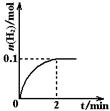
 H����OH����ƽ�� �ƶ�����������ҡ����ߡ�����������Ҫ��С����H2�����ʶ��ֲ�Ӱ�����H2��������Ӧ�������м��������Լ��е� ��
H����OH����ƽ�� �ƶ�����������ҡ����ߡ�����������Ҫ��С����H2�����ʶ��ֲ�Ӱ�����H2��������Ӧ�������м��������Լ��е� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
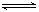 7N2��12H2O�ɴ���NO2����ת��3.6mol����ʱ�����ɵ�N2�ڱ�״������ L��
7N2��12H2O�ɴ���NO2����ת��3.6mol����ʱ�����ɵ�N2�ڱ�״������ L��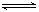 2SO3(g) ��H =" ��196.6" kJ��mol-1
2SO3(g) ��H =" ��196.6" kJ��mol-1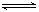 SO3(g)+NO(g) ��H = ��41.8kJ��mol-1
SO3(g)+NO(g) ��H = ��41.8kJ��mol-1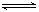 2NO2(g)�� ��H =" _________" kJ��mol-1
2NO2(g)�� ��H =" _________" kJ��mol-1 CH3OH��g������ƽ����ø����Ũ�����£�
CH3OH��g������ƽ����ø����Ũ�����£�| ���� | CO | H2 | CH3OH |
| Ũ��(mol?L��1) | 0.9 | 1.0 | 0.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
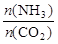 ��4ʱ��CO2��ת������ʱ��ı仯��ϵ����ͼ��ʾ��
��4ʱ��CO2��ת������ʱ��ı仯��ϵ����ͼ��ʾ��
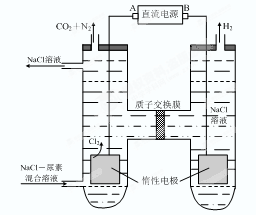
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����¯�г�����CaSO4����Na2CO3��Һ���ݺ��ٽ���������ϡ�����ܽ�ȥ�� |
| B�����ˮ�еμ�FeCl3������Һ�Ʊ�Fe(OH)3�����ԭ���Ǽ��ȴٽ���Fe3+ˮ�� |
| C����ˮ�м���������¶���ʹˮ�����ӻ���С������ƽ�������ƶ� |
| D����Ӧ2A(g) + B(g) =" 3C" (s) + D(g)��һ�����������Է����У�˵���÷�Ӧ�Ħ�H>0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
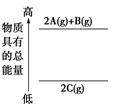
| A���٢ۢ� | B���ڢܢ� |
| C���٢ۢ� | D���ۢݢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com