
£Ø10·Ö£©³£ĪĀĻĀÓŠÅØ¶Č¾łĪŖ0£®05 mol/LµÄĖÄÖÖČÜŅŗ£ŗ¢ŁNa2CO3 ¢ŚNaHCO3 ¢ŪHCl ¢ÜNH3”¤H2O£¬»Ų“šĻą¹ŲĪŹĢā£ŗ£Ø1£©ÉĻŹöČÜŅŗÖŠ£¬æÉ·¢ÉśĖ®½āµÄŹĒ £ØĢīŠņŗÅ£©
£Ø2£©ÉĻŹöČÜŅŗÖŠ£¬¼ČÄÜÓėNaOHČÜŅŗ·“Ó¦£¬ÓÖÄÜÓėH2SO4ČÜŅŗ·“Ó¦µÄČÜŅŗÖŠ£¬Ąė×ÓÅØ¶Č“óŠ”µÄ¹ŲĻµ
£Ø3£©Ļņ¢ÜÖŠ¼ÓČėÉŁĮæNH4Cl¹ĢĢ壬“ĖŹ±c(NH4£«/OH£)µÄÖµ £Ø”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”± £©
£Ø4£©Čō½«¢ŪŗĶ¢ÜµÄČÜŅŗ»ģŗĻŗó£¬ČÜŅŗĒ”ŗĆ³ŹÖŠŠŌ£¬Ōņ»ģŗĻĒ°¢ŪµÄĢå»ż ¢ÜµÄĢå»ż£Ø”°“óÓŚ”±”¢ ”°Š”ÓŚ”±»ņ”°µČÓŚ”±£©
£Ø5£©Č”10 mLµÄ¢ŪČÜŅŗ£¬¼ÓĖ®Ļ”ŹĶµ½500 mL£¬Ōņ“ĖČÜŅŗÖŠÓÉĖ®µēĄė³öµÄc(H£«)£½ mol/L
£Ø1£©¢Ł¢Ś
£Ø2£©c(Na£«)£¾c (HCO3£) £¾c (OH£) £¾c (H£«)£¾c (CO32£)
£Ø3£©Ōö“ó
£Ø4£©Š”ÓŚ
£Ø5£©1”Į10£11
”¾½āĪö”æ
ŹŌĢā·ÖĪö£ŗ£Ø1£©ČõĖįŃĪ»ņČõ¼īŃĪ»ņČõĖįČõ¼īŃĪæÉ·¢ÉśĖ®½ā”£
£Ø2£©¼ČÄÜÓėNaOHČÜŅŗ·“Ó¦£¬ÓÖÄÜÓėH2SO4ČÜŅŗ·“Ó¦µÄČÜŅŗŹĒ¢ŚNaHCO3ČÜŅŗ£¬NaHCO3µēĄėNaHCO3£½Na++HCO3-, HCO3-Ąė×ÓĖ®½āHCO3-+H2OØPH2CO3+OH-, HCO3-Ąė×ÓÓÖµēĄėHCO3-£½H++CO32- ,Ė®½ā“óÓŚµēĄė£¬ČÜŅŗ³Ź¼īŠŌ£¬OH- ÅØ¶Č“óÓŚH+ÅØ¶Č£¬Ōņø÷Ąė×ÓÅØ¶ČµÄ“óŠ”¹ŲĻµĪŖc(Na£«)£¾c (HCO3£) £¾c (OH£) £¾c (H£«)£¾c (CO32£)
£Ø3£©¢ÜÖŠ“ęŌŚµēĄėĘ½ŗāNH3?H2OØPNH4++OH-,¼ÓČėÉŁĮæNH4Cl¹ĢĢ壬NH4+ÅضČŌö“ó£¬ŅÖÖĘĮĖ°±µÄµēĄė£¬OH-ÅØ¶Č¼õŠ”£¬c(NH4£«/OH£)µÄÖµŌö“ó£»
£Ø4£©Čō½«¢ŪŗĶ¢ÜµÄČÜŅŗµČĢå»ż»ģŗĻ£¬ÓÉÓŚ°±ŃĪµÄĖ®½āČÜŅŗ³ŹĖįŠŌ£¬ČōČÜŅŗĒ”ŗĆ³ŹÖŠŠŌ£¬ĖįµÄĢå»żŠ”Ņ»Š©£¬Ōņ»ģŗĻĒ°¢ŪµÄĢå»żŠ”ÓŚ¢ÜµÄĢå»ż£»
£Ø5£©Č”10 mLµÄ¢ŪČÜŅŗ£¬¼ÓĖ®Ļ”ŹĶµ½500 mL£¬c(HCl)£½0.05mol/l”Ā£Ø500ml/10ml£©=0.001mol/LŌņ“ĖČÜŅŗÖŠÓÉĖ®µēĄė³öµÄc(H£«)µČÓŚÓÉĖ®µēĄė³öµÄc(OH-)£½10-14/0.001mol/L£½10-11 mol/L
æ¼µć£ŗµē½āÖŹČÜŅŗ”¢ŃĪµÄĖ®½ā”¢Ė®µÄµēĄė



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014Ēļ¹óÖŻŹ”×ńŅåŹŠø߶žÉĻѧʌʌ֊»Æѧ£ØĪÄ£©ŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ĻĀĮŠ¹ŲÓŚĄ¬»ų“¦ĄķµÄ·½·Ø²»ÕżČ·µÄŹĒ
A£®“¦Ąķ³£ÓƵķ½·ØŹĒĪĄÉśĢīĀń”¢¶Ń·ŹŗĶ·ŁÉÕ
B£®½«Ą¬»ų·ÖĄą²¢»ŲŹÕŹĒ“¦ĄķµÄ·¢Õ¹·½Ļņ
C£®ĢīĀńĄ¬»ų²»ŠčŅŖÖĪĄķ£¬Ö»ŠčÉīĀń¼“æÉ
D£®·ŁÉÕĄ¬»ų»į²śÉś“óĮæĪŪČ¾æÕĘųµÄĪļÖŹ£¬¹Ź²»ŅĖ²ÉÓĆ“Ė·Ø
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014Ēļ¹óÖŻŹ”×ńŅåŹŠø߶žÉĻѧʌʌ֊»Æѧ£ØĪÄ£©ŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ĘĻĢŃĢĒ£ØC6H12O6£©ÖŠĢ¼µÄÖŹĮæ·ÖŹżŹĒ
A£®40% B£®6£®7% C£®53% D£®60%
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014Ēļø£½ØŹ”ø߶žÉĻѧʌʌ֊»Æѧ£ØĪÄ£©ŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ŹµŃéŹŅÖŠÅäÖĘ250 mL 0.5mo1”¤L-1ŃĪĖįŹ±£¬²»ŠčŅŖÓƵ½µÄŅĒĘ÷ŹĒ
A£®¾Ę¾«µĘ B£®²£Į§°ō C£®ČŻĮæĘæ D£®½ŗĶ·µĪ¹Ü
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014Ēļø£½ØŹ”ø߶žÉĻѧʌʌ֊»Æѧ£ØĪÄ£©ŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
æÉŅŌĶعżĮ½ÖÖµ„ÖŹÖ±½Ó»ÆŗĻÉś³ÉµÄĪļÖŹŹĒ
A£®SO3 B£®NO2 C£®CO2 D£®FeCl2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014Ēļŗž±±Ź”ĪäŗŗŹŠø߶žÉĻѧʌʌ֊ĮŖæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ĻĀĮŠÓŠ¹Ų³ĮµķČܽāĘ½ŗāµÄĖµ·ØÕżČ·µÄŹĒ
A£®Ksp (AB2)Š”ÓŚKsp (CD)£¬ŌņAB2µÄČܽā¶ČŠ”ÓŚCDµÄČܽā¶Č
B£®ŌŚAgClµÄ³ĮµķČܽāĘ½ŗāĢåĻµÖŠ£¬¼ÓČėÕōĮóĖ®£¬AgClµÄKspŌö“ó
C£®ŌŚAgIµÄ³ĮµķČܽāĘ½ŗāĢåĻµÖŠ£¬¼ÓČėK2S¹ĢĢ壬AgI³ĮµķæÉ×Ŗ»ÆĪŖAg2S³Įµķ
D£®ŌŚCaCO3µÄ³ĮµķČܽāĘ½ŗāĢåĻµÖŠ£¬ĶØČėCO2ĘųĢ壬ČܽāĘ½ŗā²»ŅʶÆ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014Ēļŗž±±Ź”ĪäŗŗŹŠø߶žÉĻѧʌʌ֊ĮŖæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ŅŃÖŖ£ŗ HCl(aq)ÓėNaOH(aq)·“Ó¦µÄ?H£½ £55.6 kJ/mol£» HCN(aq)ÓėNaOH(aq)·“Ó¦µÄ ?H£½ £12.1 kJ/mol”£ŌņHCNŌŚĖ®ČÜŅŗÖŠµēĄėµÄ?HµČÓŚ
A£®£« 43.5 kJ/mol B£®£67.7 kJ/mol
C£®£«67.7 kJ/mol D£®£43.5 kJ/mol
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014Ēļŗž±±Ź”ĪäŗŗŹŠøßŅ»ÉĻѧʌʌ֊ĮŖæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ĻĀĮŠ¹ŲÓŚĪļÖŹµÄĮæÅØ¶ČµÄ±ķŹöÖŠÕżČ·µÄŹĒ
A£®0.3 mol/L Na2SO4ČÜŅŗÖŠŗ¬ÓŠNa+ ŗĶSO42£×ÜĪļÖŹµÄĮæĪŖ0.9mol
B£®µ±1LĖ®ĪüŹÕ22.4LNH3Ź±ĖłµĆ°±Ė®µÄÅØ¶Č²»ŹĒ1 mol/L£¬Ö»ÓŠµ±22.4L NH3ČÜÓŚĖ®ÖʵĆ1L°±Ė®Ź±£¬ĘäÅØ¶Č²ÅŹĒ1 mol/L
C£®10”ꏱ0.5 mol/LµÄĻ”ŃĪĖį100mL£¬Õō·¢µō5gĖ®£¬ĄäČ“µ½10”ꏱ£¬ĘäĢå»żŠ”ÓŚ100mL£¬ĖüµÄĪļÖŹµÄĮæÅØ¶Č“óÓŚ0.5 mol/L
D£®10”ꏱ0.5 mol/LµÄKCl±„ŗĶČÜŅŗ100mL£¬Õō·¢µō5gĖ®£¬ĄäČ“µ½10”ꏱ£¬ĘäĢå»żŠ”ÓŚ100 mL£¬ĖüµÄĪļÖŹµÄĮæÅضČČŌĪŖ0.5 mol/L
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014ĒļÕć½Ź”ŗ¼ÖŻµŲĒųĮłŠ£øßŅ»ÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø4·Ö£©ĻĀĶ¼±ķŹ¾ĀČ»ÆÄĘČÜÓŚĖ®ŗóŠĪ³ÉĀČ»ÆÄĘČÜŅŗµÄŹ¾ŅāĶ¼”£
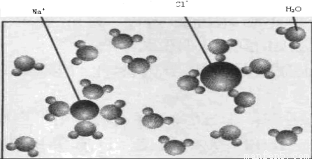
Ķ¼ÖŠæņÄŚNaClČÜŅŗÖŠNaClÓėĖ®·Ö×ÓµÄĪļÖŹµÄĮæÖ®±ČĪŖ £¬
ĀČ»ÆÄĘČÜŅŗµÄÖŹĮæ·ÖŹżĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com