
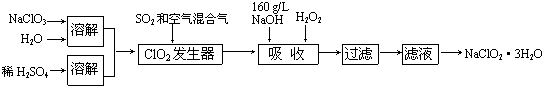
·ÖĪö ¹żŃõ»ÆĒā·ØÉś²śŃĒĀČĖįÄĘ£¬ÓÉĮ÷³ĢæÉÖŖ£¬NaClO3ČܽāŗóÓėĮņĖį·¢ÉśŃõ»Æ»¹Ō·“Ӧɜ³ÉClO2£¬½įŗĻŠÅĻ¢¢ŚæÉÖŖ»ģŗĻĘųĢåĻ”ŹĶClO2£¬ĪüŹÕĖžÄŚ·¢Éś2NaOH+2ClO2+H2O2ØT2NaClO2+2H2O+O2£¬¹żĀĖŗ󣬽įŗĻŠÅĻ¢¢ŁæÉÖŖ£¬ĀĖŅŗÕō·¢ÅØĖõ”¢ĄäČ“½į¾§”¢¹żĀĖ”¢Ļ“µÓ”¢øÉŌļµĆµ½NaClO2•3H2O£¬ŅŌ“ĖĄ“½ā“š£®
½ā“š ½ā£ŗ¹żŃõ»ÆĒā·ØÉś²śŃĒĀČĖįÄĘ£¬ÓÉĮ÷³ĢæÉÖŖ£¬NaClO3ČܽāŗóÓėĮņĖį·¢ÉśŃõ»Æ»¹Ō·“Ӧɜ³ÉClO2£¬½įŗĻŠÅĻ¢¢ŚæÉÖŖ»ģŗĻĘųĢåĻ”ŹĶClO2£¬ĪüŹÕĖžÄŚ·¢Éś2NaOH+2ClO2+H2O2ØT2NaClO2+2H2O+O2£¬¹żĀĖŗ󣬽įŗĻŠÅĻ¢¢ŁæÉÖŖ£¬ĀĖŅŗÕō·¢ÅØĖõ”¢ĄäČ“½į¾§”¢¹żĀĖ”¢Ļ“µÓ”¢øÉŌļµĆµ½NaClO2•3H2O£¬
£Ø1£©ÓÉĢāÄæÖŠµÄŠÅĻ¢æÉÖŖ£¬“æClO2Ņ×·Ö½ā±¬ÕØ£¬ĖłŅŌĶØČėæÕĘųµÄÄæµÄŹĒĻ”ŹĶClO2£¬·ĄÖ¹·¢Éś±¬ÕØ£¬
¹Ź“š°øĪŖ£ŗĻ”ŹĶClO2£¬·ĄÖ¹·¢Éś±¬ÕØ£»
£Ø2£©ĪüŹÕĖžÖŠ·¢ÉśµÄŹĒ¶žŃõ»ÆĀČÓėĒāŃõ»ÆÄĘ”¢¹żŃõ»ÆĒā·¢Éś·“Ӧɜ³ÉŃĒĀČĖįÄĘ£ØNaClO2£©£¬ClŌŖĖŲµÄ»ÆŗĻ¼Ū½µµĶ£¬Ōņ¹żŃõ»ÆĒāÖŠµÄOŌŖĖŲµÄ»ÆŗĻ¼ŪÉżøߣ¬ĖłŅŌ²śĪļŌŚ»¹ÓŠŃõĘųÉś³É£¬øł¾ŻŌŖĖŲŹŲŗćæÉÖŖ²śĪļÖŠÓŠĖ®Éś³É£¬ĖłŅŌ»Æѧ·½³ĢŹ½ŹĒ2NaOH+2ClO2+H2O2ØT2NaClO2+2H2O+O2£¬
¹Ź“š°øĪŖ£ŗ2NaOH+2ClO2+H2O2ØT2NaClO2+2H2O+O2£»
£Ø3£©¹żŃõ»ÆĒāŹÜČČŅ×·Ö½ā£¬ĖłŅŌĪüŹÕĖžµÄĪĀ¶Č²»Äܳ¬¹ż20”ę£¬¹Ź“š°øĪŖ£ŗ·ĄÖ¹H2O2·Ö½ā£»
£Ø4£©Ń”ŌńŗĻŹŹµÄ»¹Ō¼Į²»ÄÜŅżČėĘäĖūŌÓÖŹ£¬»¹ŌŠŌŅŖŹŹÖŠ£¬»¹ŌŠŌĢ«Ē棬»į½«ClO2»¹ŌĪŖøüµĶ¼ŪĢ¬²śĪļ£¬Ó°ĻģNaClO2Éś²ś£»ĒŅ·½±ćŗóŠų·ÖĄėĢį“棬¼ÓČėŹŌ¼Į²»ÄÜøÉČÅŗóŠųÉś²ś£¬Na2O2ČÜÓŚĖ®Ļąµ±ÓŚH2O2£¬Na2S”¢FeCl2»¹ŌŠŌ½ĻĒæ£¬Éś³ÉĪļÓėNaClO2·ÖĄė±Č½ĻĄ§ÄŃ£¬¹Ź“š°øĪŖ£ŗa£»
£Ø5£©“ÓČÜŅŗÖŠµĆµ½ŗ¬½į¾§Ė®µÄ¾§Ģ壬ֻÄܲÉČ”Õō·¢”¢ÅØĖõ”¢ĄäČ“½į¾§·½·Ø£¬Ķعż¹żĀĖµĆµ½“Ö¾§Ģ壬¹Ź“š°øĪŖ£ŗÕō·¢ÅØĖõ£»ĄäČ“½į¾§£®
µćĘĄ ±¾Ģāæ¼²éĪļÖŹµÄÖʱøŹµŃ飬ĪŖøßĘµæ¼µć£¬°ŃĪÕÖʱøŹµŃéŌĄķ”¢Į÷³ĢÖŠµÄ·“Ó¦”¢»ģŗĻĪļ·ÖĄėĢį“æĪŖ½ā“šµÄ¹Ų¼ü£¬²ąÖŲ·ÖĪöÓėÓ¦ÓĆÄÜĮ¦µÄ漲飬עŅāŃõ»Æ»¹Ō·“Ó¦µÄÓ¦ÓĆ£¬ĢāÄæÄŃ¶Č²»“ó£®


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
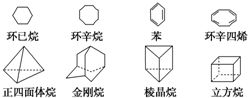
 £Ø1£¬4-¶ž¼×±½£©”¢
£Ø1£¬4-¶ž¼×±½£©”¢ £Ø1£¬3£¬5-Čż¼×±½£©£®
£Ø1£¬3£¬5-Čż¼×±½£©£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | µ„Ļ©Ģž | B£® | ±„ŗĶŅ»ŌŖ“¼ | C£® | ±„ŗĶŅ»ŌŖČ© | D£® | ±„ŗĶŅ»ŌŖōČĖį |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| “ĪĀČĖį | Į×Ėį | ĮņĖį | øßĀČĖį | |
| ŗ¬ŃõĖį |  |  |  | 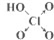 |
| ·ĒōĒ»łŃõŌ×ÓŹż | 0 | 1 | 2 | 3 |
| ĖįŠŌ | ČõĖį | ÖŠĒæĖį | ĒæĖį | ×īĒæĖį |
 £®ŃĒĮ×ĖįÓė¹żĮæµÄĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖH3PO3+2NaOHØTNa2HPO3+2H2O£®
£®ŃĒĮ×ĖįÓė¹żĮæµÄĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖH3PO3+2NaOHØTNa2HPO3+2H2O£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¾łČÜÓŚĖ® | B£® | ¾łĪŖ°×É«¹ĢĢå | ||
| C£® | ŹÜČČ¾łŅ×·Ö½ā | D£® | ĘäĖ®ČÜŅŗ¾łÄÜŹ¹·ÓĢŖČÜŅŗ±äŗģ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓŠĖ®²Ī¼ÓµÄÓŠ»ś»Æѧ·“Ó¦¶¼æÉŅŌ½Š×öĖ®½ā·“Ó¦ | |
| B£® | ±ūĻ©”¢ĀČŅŅĻ©”¢±½·Ö×ÓÄŚĖłÓŠŌ×Ó¾łŌŚĶ¬Ņ»Ę½Ćę | |
| C£® | ĆŽ»ØŗĶľ²ÄŌŚÖ÷ŅŖ³É·ÖŹĒĻĖĪ¬ĖŲ£¬ŃņĆ«ŗĶ²ĻĖæµÄÖ÷ŅŖ³É·ÖŹĒµ°°×ÖŹ | |
| D£® | ÕįĢĒŹĒ·Ö²¼×ī¹ćµÄ¶žĢĒ£¬Ņ»·Ö×ÓÕįĢĒÄÜĖ®½āĪŖĮ½·Ö×ÓĘĻĢŃĢĒ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | F-µÄ½į¹¹Ź¾ŅāĶ¼£ŗ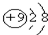 | B£® | ŅŅĻ©µÄ½į¹¹¼ņŹ½CH2CH2 | ||
| C£® | NaClµÄµē×ÓŹ½£ŗ | D£® | N2µÄ½į¹¹Ź½£ŗ£ŗN”ŌN£ŗ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com