
��1��4.6 g CH3CH2OH��ȫȼ������ˮ�Ͷ�����̼����___________mol����ת�ơ�
��2����A�����͵���Ҫ�ɷ�֮һ��ȡ11.4 g��A������һ������������У���ȼʹ֮��ַ�Ӧ�ָ�����״�������������x L����ʣ�����徭����ʯ�����գ���ʯ����������y g�����ݼ��±�����������������ڱ�״���²ⶨ����
| �������Ϊ20 L | �������Ϊ30 L | �������Ϊ40 L |
x | 2.22 | 10.08 | 10.08 |
y | 4.3 | 35.2 | 35.2 |
11.4 g��A�к�̼�����ʵ�����_______________mol����������ʵ�����_____________mol�������ķ���ʽ��_____________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)4.6 g CH3CH2OH��ȫȼ������ˮ�Ͷ�����̼���� mol����ת�ơ�?
(2)��A�����͵���Ҫ�ɷ�֮һ��ȡ11.4g��A������һ������������У���ȼʹ֮��ַ�Ӧ�ָ�����״�������������x L����ʣ�����徭����ʯ�����գ���ʯ����������yg�����ݼ��±�(��������������ڱ�״���²ⶨ)��
| �������Ϊ20 L | �������Ϊ30 L | �������Ϊ40 L |
x | 2.22 | 10.08 | 10.08 |
y | 4.3 | 35.2 | 35.2 |
11.4 g��A�к�̼�����ʵ����� mol����������ʵ����� mol�������ķ���ʽ�� ��?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��ʯ����ֽ(��������)����?
�ڵζ��ܣ� ��?
�ۼ���ƿ(�ռ�����)�� ��?
��������ƽ ��?
(��)Ŀǰ�����ͼ۾Ӹ߲��¡��Ҵ�������ָ�������м���10%���Ҵ�(�����)���Ҵ����;�������ֵ�ߡ������Ժõ��ص㣬�ƹ�ʹ�ÿɻ�����Դ��ȱ���ٽ����÷�չ�����������ӵ��Ҵ����ܺ�ˮ�������Ӱ�췢����������ת��ʹ���������Ʊ���ˮ�Ҵ��ɲ�ȡ���·�����??
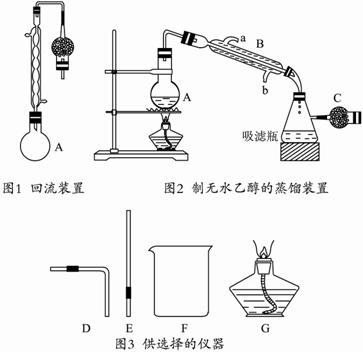
����250 mLԲ����ƿ�м���95%���Ҵ�100 mL�����Ƶ���ʯ��30 g����ˮԡ�м��Ȼ���1��2Сʱ��(��ͼ1��ʾ)?
��ȡ�������ܣ��ij���ͼ2��ʾ��װ�ã��ٽ�A����ˮԡ������?
�۰����������5 mL���Һ������ա�?
���ú�ɵ�����ƿ��Ϊ�����������ܽ�һ֧װ��CaCl2�ĸ����C��ʹ���������ͨ��������Һ�γ���Ϊֹ������99.5%�ľƾ����Իش�?
(1)��֪��101 kPa��25 ��ʱ��?
C2H5OH(l)+3O2(g)=2CO2(g)+3H2O(l);��H =-1 367 kJ��mol-1??
1 g�Ҵ���ȫȼ������Һ̬ˮʱ�ų� ������?
(2)ͼ2�еĸ����C�������� ������B�������� ������ˮ�Ǵӿ� (�a����b��)���������ܡ�?
(3)��ˮCaCl2��������ˮ��������ƿA���ܷ�����ˮCaCl2������ʯ�ң� (��д���ܡ������ܡ�)��ԭ���� ��?
(4)�������ò�Ʒ���Ƿ�ˮ�IJ�������������������������������������������������
(5)ijͬѧ��Ҫ����ͼ2װ�õ������ԣ����ɴ�ͼ3��ѡ���ļ�������(����������) ����������������ԵIJ������� ��?
(6)д����������ת��Ϊ�ƾ��Ļ�ѧ����ʽ�� ��?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009�����ʡ�Ƹ��л�����ѧ�߿���ѧ��ģ�Ծ��������棩 ���ͣ�ѡ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com