
| t/min | X/mol | Y/mol | Z/mol |
| 0 | 1.00 | 1.00 | 0.00 |
| 1 | 0.90 | 0.80 | 0.20 |
| 3 | 0.75 | 0.50 | 0.50 |
| 5 | 0.65 | 0.30 | 0.70 |
| 9 | 0.55 | 0.10 | 0.90 |
| 10 | 0.55 | 0.10 | 0.90 |
| 14 | 0.55 | 0.10 | 0.90 |

���� ��1�����ݱ������ݣ�������ʱ��ʱX��Y��Z��Ӧ�ĵ㣬��Բ���������������ɣ���Ӧ��9minʱ����ƽ�⣻
��2��9min����ƽ�⣬ת����XΪ��1.00-0.55��mol=0.45mol����������X��ת���ʣ�
��3����0��1min�ڣ���n��X������n��Y������n��Z��=��1-0.9��mol����1-0��8��mol��0.2mol=1��2��2����֪��ѧ����ʽΪX+2Y?2Z��
���ߢ١��ڡ��۵���ƽ��ʱ�����ԭƽ��̣���Ӧ���ʼӿ죬��Ӧ�¶ȡ�ѹǿ����Ӧ�������¶ȡ�����ѹǿ��ʹ�ô�����Ӱ��ƽ���ƶ���Z�����ʵ�����ԭƽ����ȣ�����ӦΪ���������С�ķ��ȷ�Ӧ�������¶�ƽ�������ƶ���ƽ��ʱZ�����ʵ�����ԭƽ��С��������ѹǿƽ�������ƶ���ƽ��ʱZ�����ʵ�����ԭƽ���
��� �⣺��1�����ݱ������ݣ���Ӧ��9minʱ����ƽ�⣬������ʱ��ʱX��Y��Z��Ӧ�ĵ㣬��Բ���������������ɣ�X��Y��Z�����ʵ�����n����ʱ�䣨t���仯�����ߣ�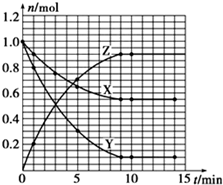 ��
��
�ʴ�Ϊ�� ��
��
��2��9min����ƽ�⣬ת����XΪ��1.00-0.55��mol=0.45mol����X��ת����Ϊ$\frac{0.45mol}{1mol}$��1005=45%��
�ʴ�Ϊ��45%��
��3����0��1min�ڣ���n��X������n��Y������n��Z��=��1-0.9��mol����1-0��8��mol��0.2mol=1��2��2����֪��ѧ����ʽΪX+2Y?2Z��
���ߢ١��ڡ��۵���ƽ��ʱ�����ԭƽ��̣���Ӧ���ʼӿ죬��Ӧ�¶ȡ�ѹǿ����Ӧ�������¶ȡ�����ѹǿ��ʹ�ô�����Ӱ��ƽ���ƶ���Z�����ʵ�����ԭƽ����ȣ�����ӦΪ���������С�ķ��ȷ�Ӧ�������¶�ƽ�������ƶ���ƽ��ʱZ�����ʵ�����ԭƽ��С��������ѹǿƽ�������ƶ���ƽ��ʱZ�����ʵ�����ԭƽ���
���ߢ�ƽ��ʱ����Z������ԭƽ��С�������ߢٸı�������������¶ȣ�
���ߢڵ���ƽ��Z�����ʵ���Ϊ0.9mol����ԭƽ����ȣ����ߢڸı��������ʹ�ô�����
���ߢ۵���ƽ��Z�����ʵ���Ϊ0.95mol����ԭƽ������ߢ۸ı������������ѹǿ��
�ʴ�Ϊ�������¶ȣ�ʹ�ô���������ѹǿ��
���� ���⿼�黯ѧƽ�������Ӱ�����ء���ѧƽ��ͼ�ؼ���ȷ����Ӧ����ʽ�����ؿ���ѧ��������������������


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ɫ��Һ�У�NH4+��Fe2+��SO42-��CO32- | |
| B�� | �ں��д���Ba2+ ����Һ�У�NH4+��Na+��Cl-��CO32- | |
| C�� | ��ǿ������Һ�У�Na+��Cl-��K+��SO42- | |
| D�� | ��ǿ������Һ�У�K+��Fe2+��Cl-��HCO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ӽ��ܽǶȿ��ǣ���ҵѡ����ˮ�Ȼ�þΪԭ��ұ��þ����ѡ������þ | |
| B�� | ����������Ȼ�þʱ������ˮ��þ������ˮ������Ӧ | |
| C�� | ���������ò����ý���ͭ���� | |
| D�� | ��ʪ���KI������ֽ���Լ��������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
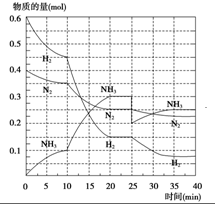 ������Ҫ�ĵ��ʣ��ϳ�ԭ��Ϊ��N2��g��+3H2��g�����¡���ѹ����
������Ҫ�ĵ��ʣ��ϳ�ԭ��Ϊ��N2��g��+3H2��g�����¡���ѹ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| ѡ�� | ʵ�� | ���ͻ���� |
| A | �ýྻ��Pt˿պȡij��Һ������ɫ��Ӧ������ʻ�ɫ | ����Һ��һ��������K+ |
| B | �ýྻ�IJ����������Na2O2����֬��������֬��ȼ�� | CO2��H2O��Na2O2��Ӧ�Ƿ��ȷ�Ӧ |
| C | ��ij��Һ�еμ������ữ��Ba��NO3��2��Һ������ɫ���� | ����Һ�к���SO42- |
| D | ����һ��ǿ������������Һ�����������ġ�ͨ·���� | ����һ��������ˮ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������������������������� | B�� | ����Ȼ���ˮ��Һ | ||
| C�� | ������������������Ӧ | D�� | �������ᷴӦ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com