
| A£® | NaHCO3ČÜŅŗÖŠ£ŗc£ØH+£©+c£ØNa+£©ØTc£ØOH-£©+c£ØCO32-£©+c£ØHCO3-£© | |
| B£® | pH=3µÄCH3COOHČÜŅŗÓėpH=11µÄNaOHČÜŅŗµČĢå»ż»ģŗĻŗóµÄČÜŅŗÖŠ£ŗc£ØOH-£©£¾c£ØH+£© | |
| C£® | 0.1mol/LµÄNH4ClČÜŅŗÖŠ£ŗc£ØCl-£©£¾c£ØH+£©£¾c£ØNH4+£©£¾c£ØOH-£© | |
| D£® | ĪļÖŹµÄĮæÅضČĻąµČµÄCH3COOHŗĶCH3COONaČÜŅŗµČĢå»ż»ģŗĻŗóµÄČÜŅŗÖŠ£ŗ2c£ØNa+£©ØTc£ØCH3COOH£©+c£ØCH3COO-£© |
·ÖĪö A£®øł¾ŻĢ¼ĖįĒāÄĘČÜŅŗÖŠµÄµēŗÉŹŲŗćÅŠ¶Ļ£»
B£®“×ĖįĪŖČõĖį£¬»ģŗĻŅŗÖŠ“×Ėį¹żĮ棬ČÜŅŗ³ŹĖįŠŌ£¬Ōņc£ØOH-£©£¼c£ØH+£©£»
C£®ļ§øłĄė×ÓµÄĖ®½ā³Ģ¶Č½ĻŠ”£¬Ōņc£ØNH4+£©£¾c£ØH+£©£»
D£®øł¾Ż»ģŗĻŅŗÖŠµÄĪļĮĻŹŲŗćÅŠ¶Ļ£®
½ā“š ½ā£ŗA£®NaHCO3ČÜŅŗÖŠ£¬øł¾ŻµēŗÉŹŲŗćæÉÖŖ£ŗ£ŗc£ØH+£©+c£ØNa+£©ØTc£ØOH-£©+2c£ØCO32-£©+c£ØHCO3-£©£¬¹ŹA“ķĪó£»
B£®pH=3µÄCH3COOHČÜŅŗÓėpH=11µÄNaOHČÜŅŗµČĢå»ż»ģŗĻ£¬ÓÉÓŚ“×ĖįĪŖČõĖį£¬Ōņ“×Ėį¹żĮ棬ČÜŅŗĻŌŹ¾ĖįŠŌ£ŗc£ØOH-£©£¼c£ØH+£©£¬¹ŹB“ķĪó£»
C£®0.1mol/LµÄNH4ClČÜŅŗÖŠ£¬ļ§øłĄė×ÓĖ®½ā£¬ČÜŅŗ³ŹĖįŠŌ£¬ÓÉÓŚĖ®½ā³Ģ¶Č½ĻŠ”£¬Ōņc£ØNH4+£©£¾c£ØH+£©£¬ÕżČ·µÄĄė×ÓÅØ¶Č“óŠ”ĪŖ£ŗc£ØCl-£©£¾c£ØNH4+£©£¾c£ØH+£©£¾c£ØOH-£©£¬¹ŹC“ķĪó£»
D£®ĪļÖŹµÄĮæÅضČĻąµČµÄCH3COOHŗĶCH3COONaČÜŅŗµČĢå»ż»ģŗĻ£¬øł¾ŻĪļĮĻŹŲŗćæÉµĆ£ŗ2c£ØNa+£©ØTc£ØCH3COOH£©+c£ØCH3COO-£©£¬¹ŹDÕżČ·£»
¹ŹŃ”D£®
µćĘĄ ±¾Ģāæ¼²éĮĖĄė×ÓÅØ¶Č“óŠ”±Č½Ļ£¬ĢāÄæÄѶČÖŠµČ£¬Ć÷Č·ŃĪµÄĖ®½āŌĄķ¼°ĘäÓ°ĻģĪŖ½ā“š¹Ų¼ü£¬×¢ŅāÕĘĪÕµēŗÉŹŲŗć”¢ĪļĮĻŹŲŗćµÄŗ¬Ņå¼°Ó¦ÓĆ·½·Ø£¬ŹŌĢāÅąŃųĮĖѧɜµÄ·ÖĪöÄÜĮ¦¼°Įé»īÓ¦ÓĆÄÜĮ¦£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĻņNaOHČÜŅŗÖŠÖšµĪµĪČė±„ŗĶĀČ»ÆĢśČÜŅŗĄ“ÖʱøFe£ØOH£©3½ŗĢå | |
| B£® | ĻņFe£ØOH£©3½ŗĢåÖŠÖšµĪµĪČėĻ”ŃĪĖį£¬ĻÖĻóŹĒĻČ³öĻÖŗģŗÖÉ«³Įµķ£¬ŗóČܽā×ŖĪŖ»ĘÉ«ČÜŅŗ | |
| C£® | ”°¶”“ļ¶ū”±Š§Ó¦ŹĒĒų·Ö½ŗĢåŗĶČÜŅŗµÄĪØŅ»ŹÖ¶Ī | |
| D£® | ¾²µē³ż³¾Óė½ŗĢåŠŌÖŹĪŽ¹Ų |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā

| A£® | ŅĄ¾ŻĶ¼¼×æÉÅŠ¶ĻÕż·“Ó¦ĪŖ·ÅČČ·“Ó¦ | |
| B£® | ŌŚĶ¼ŅŅÖŠ£¬ŠéĻßæɱķŹ¾Ź¹ÓĆĮĖ“߻ƼĮ | |
| C£® | ČōÕż·“Ó¦µÄ”÷H£¼0£¬Ķ¼±ūæɱķŹ¾ÉżøßĪĀ¶ČŹ¹Ę½ŗāĻņÄę·“Ó¦·½ĻņŅĘ¶Æ | |
| D£® | ÓÉĶ¼¶”ÖŠĘųĢåĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæĖęĪĀ¶ČµÄ±ä»ÆĒéæö£¬æÉĶĘÖŖÕż·“Ó¦µÄ”÷H£¾0 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | SO42- | B£® | Fe2+ | C£® | S2- | D£® | OH- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
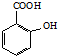 £©·“Ó¦ÄܵƵ½»ÆѧŹ½ĪŖC7H5O3NaµÄŹĒA
£©·“Ó¦ÄܵƵ½»ÆѧŹ½ĪŖC7H5O3NaµÄŹĒA²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | N2 | B£® | MgCl2 | C£® | Na2O | D£® | Na2O2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
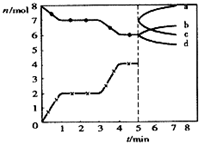 ŌŚĢå»żĪŖ2LµÄĆܱÕČŻĘ÷ÖŠ½ųŠŠĻĀĮŠ·“Ó¦£ŗC£Øs£©+CO2£Øg£©ØT2CO£Øg£©£»”÷H=+Q kJ•mol-1£®ČēĶ¼ĪŖCO2”¢COµÄĪļÖŹµÄĮæĖꏱ¼ätµÄ±ä»Æ¹ŲĻµĶ¼£®ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ£Ø””””£©
ŌŚĢå»żĪŖ2LµÄĆܱÕČŻĘ÷ÖŠ½ųŠŠĻĀĮŠ·“Ó¦£ŗC£Øs£©+CO2£Øg£©ØT2CO£Øg£©£»”÷H=+Q kJ•mol-1£®ČēĶ¼ĪŖCO2”¢COµÄĪļÖŹµÄĮæĖꏱ¼ätµÄ±ä»Æ¹ŲĻµĶ¼£®ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ£Ø””””£©| A£® | ŌŚ0-1minÄŚCOµÄĪļÖŹµÄĮæŌö¼ÓĮĖ2mol | |
| B£® | µ±¹Ģ½¹ĢæµÄÖŹĮæ²»·¢Éś±ä»ÆŹ±£¬ĖµĆ÷·“Ó¦ŅŃ“ļĘ½ŗāדĢ¬ | |
| C£® | 5minŹ±ŌŁ³äČėŅ»¶ØĮæµÄCO£¬n£ØCO£©”¢n£ØCO2£©µÄ±ä»ÆæÉ·Ö±šÓÉc”¢bĒśĻß±ķŹ¾ | |
| D£® | 3minŹ±ĪĀ¶ČÓÉT1Éżøßµ½T2£¬ÖŲŠĀĘ½ŗāŹ±K£ØT2£©Š”ÓŚK£ØT1£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĒāŃõ»ÆĀĮæÉÓĆÓŚÖĘĪøĖįÖŠŗĶ¼Į | |
| B£® | Ė®²£Į§æÉÓĆÓŚÉś²śš¤ŗĻ¼ĮŗĶ·Ą»š¼Į | |
| C£® | “ĪĀČĖįÄĘČÜŅŗæÉÓĆÓŚ»·¾³µÄĻū¶¾É±¾ś | |
| D£® | ¶žŃõ»ÆĮņæɹć·ŗÓĆÓŚŹ³Ę·µÄĘÆ°× |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com