
LiPF6������ӵ���й㷺Ӧ�õĵ���ʡ�ij������LiF��PCl5Ϊԭ�ϣ����·�Ӧ�Ʊ�LiPF6�����������£�
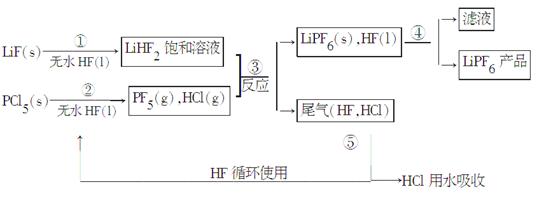
��֪��HCl�ķе��ǣ�85.0 �棬HF�ķе���19.5 �档
(1)�ڢٲ���Ӧ����ˮHF��������_______��________����Ӧ�豸�����ò������ʵ�ԭ����________________________(�û�ѧ����ʽ��ʾ)����ˮHF�и�ʴ�ԺͶ��ԣ�������ȫ�ֲ���ʾ�������С�Ľ�HFմ��Ƥ���ϣ���������2%��________��Һ��ϴ��
(2)������������ˮ�����½��У��ڢ۲���Ӧ��PF5����ˮ�⣬�����Ϊ�����ᣬд��PF5ˮ��Ļ�ѧ����ʽ��
__________________________________________________________��
(3)�ڢܲ�������õķ�����__________________���ڢݲ�����β����HF��HCl���õķ�����__________________________________��
(4)LiPF6��Ʒ��ͨ����������LiF��ȡ��Ʒw g�����Li�����ʵ���Ϊn mol�������Ʒ��LiPF6�����ʵ���Ϊ________mol(�ú�w�� n�Ĵ���ʽ��ʾ)��
n�Ĵ���ʽ��ʾ)��
������(1)�����̿�֪���ڢٲ���Ӧ����ˮHF����Ϊ��Ӧ���⣬�����ܼ������á������к���SiO2������HF������Ӧ������ʴ����ѧ����ʽΪSiO2��4HF===SiF4����2H2O��HF�������ԣ�մ��Ƥ���ϣ���ѡ�ü��Խ�����NaHCO3��Һ���г�ϴ��
(2)PF 5��PԪ���ԣ�5�ۣ�FԪ���ԣ�1�ۣ�Ǩ��Ӧ������ˮ�ⷴӦ֪ʶ�Ƴ�PF5����ˮ�ⷴӦӦ����H3PO4��HF����ѧ����ʽΪPF5��4H2O===H3PO4��5HF��
5��PԪ���ԣ�5�ۣ�FԪ���ԣ�1�ۣ�Ǩ��Ӧ������ˮ�ⷴӦ֪ʶ�Ƴ�PF5����ˮ�ⷴӦӦ����H3PO4��HF����ѧ����ʽΪPF5��4H2O===H3PO4��5HF��
(3)�ڢܲ�����õ���Һ��LiPF6���壬��Ȼ�ò�����Ϊ���ˡ�HCl��HF�ķе�ֱ�Ϊ��85.0 ���19.5�棬�ڢݲ�����β���е�HCl��HF���ɲ��������ķ�����ʹHFҺ���������Է��롣
(4)�������֪��152 g��mol��1��n(LiPF6)��26 g��mol��1��n(LiF)��w g��n(LiPF6)��n(LiF)��n mol���� ��n(LiPF6)��(w��26n)/126 mol��
��n(LiPF6)��(w��26n)/126 mol��
�𰸡�(1)��Ӧ��ܼ���SiO2��4HF===SiF4����2H2O
NaHCO3
(2)PF5��4H2O===H3PO4��5HF
(3)���ˡ�����
(4)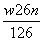


 ������������ϵ�д�
������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��0.20 mol NO��0.1 mol CO����һ���ݻ��㶨Ϊ1L���ܱ������з�����Ӧ��

�ڲ�ͬ�����£���Ӧ�����в������ʵ�Ũ�ȱ仯��ͼ��ʾ��
����˵����ȷ����
A.�����ڵ�ѹǿ�������仯˵���÷�Ӧ�ﵽƽ��
B.�����������ٳ���0.20 mol NOʱ��ƽ��������Ӧ��
���ƶ���K����
C����12 minʱ�ı�ķ�Ӧ����Ϊ�����¶�
D.��������ڳ���He��������������ѹǿ��������
��Ӧ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и�����������ã������ﲻ�淴Ӧ������Ӧ��������仯���仯����(����)
A��Na��O2 B��Na2CO3��HCl
C��AlCl3��NaOH D��NaOH��NaHCO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ȡ����Ϊ14.8 g��Na2CO3��NaHCO3�Ĺ��������100 mL 2.50 mol/L������ǡ����ȫ��Ӧ�����ٷų����塣
(1)��ԭ�������Na2CO3������������
(2)��100 mL 2.50 mol/L���������ñ�ǩ��ͼ��ʾ��Ũ�����Ƶã������Ũ���������Ƕ��٣�
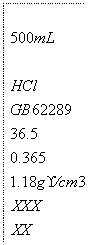
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ܱ������м���a mol����PH4I����һ�����¶��·������·�Ӧ��
PH4I(s)PH3(g)��HI(g)��
4PH3(g)P4(g)��6H2(g)��
2HI(g)H2(g)��I2(g)��
����������Ӧ����ƽ����HIΪb mol��I2Ϊc mol��H2Ϊd mol����
(1)ƽ���������P4��PH3�����ʵ�����________(�ô���ʽ��ʾ)��
(2)a��b��c������ѭ�Ĺ�ϵ��a>________(��b��c�Ĺ�ϵʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������������(����)
A����ϩ�ͱ�����ʹ��ˮ����ɫ����ɫ��ԭ����ͬ
B�����ۡ���֬�������ʶ���ˮ�⣬��ˮ����ﲻͬ
C��ú�Ϳ���ʯ�ͷ����ã�������ȼ�Ϻͱ�������������
D���Ҵ������ᡢ�����������ܷ���ȡ����Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
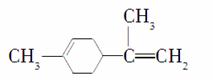 ��(2014���人�е���)����ϩ��һ��ʳ�����ϣ���ṹ��ʽ��ͼ���й�����ϩ�ķ�����ȷ����(����)
��(2014���人�е���)����ϩ��һ��ʳ�����ϣ���ṹ��ʽ��ͼ���й�����ϩ�ķ�����ȷ����(����)
A������ϩ��һ�ȴ�����7��
B������ϩ�Ͷ�������Ϊͬ���칹��
C������ϩ�ķ��������е�̼ԭ�ӿ�����ͬһ��ƽ����
D����һ�������£�����ϩ���Է����ӳɡ�ȡ������������ԭ�ȷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��Һ�п��ܺ���H����NH ��Mg2����Al3����Fe3����CO
��Mg2����Al3����Fe3����CO ��SO
��SO ��NO
��NO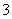 �еļ��֡���������п����������ɫ��ζ�����壻��������NaOH��Һ��������ɫ�������Ҳ����ij����������NaOH�����ʵ���֮��Ĺ�ϵ��ͼ��ʾ��������˵����ȷ����(����)
�еļ��֡���������п����������ɫ��ζ�����壻��������NaOH��Һ��������ɫ�������Ҳ����ij����������NaOH�����ʵ���֮��Ĺ�ϵ��ͼ��ʾ��������˵����ȷ����(����)
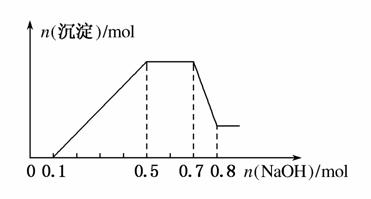
A����Һ�е�������ֻ��H����Mg2����Al3��
B����Һ��n(NH )��0.2 mol
)��0.2 mol
C����Һ��һ������CO �����ܺ���SO
�����ܺ���SO ��NO
��NO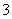
D��n(H��):n(Al3��):n(Mg2��)��1:1:1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һƿ�������Һ�����п��ܺ���NH ��K����Mg2����Ba2����Al3����Fe3����SO
��K����Mg2����Ba2����Al3����Fe3����SO ��CO
��CO ��NO
��NO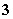 ��I����ȡ����Һ��������ʵ�飺
��I����ȡ����Һ��������ʵ�飺
(1)��pH��ֽ���飬������Һ��ǿ���ԣ�
(2)ȡ������Һ����������CCl4���������Ƶ���ˮ������CCl4���Ϻ�ɫ��
(3)��ȡ������Һ����μ���ϡNaOH��Һ��ʹ��Һ��������ת��Ϊ���ԣ��ڵμӹ����У���Һ�о��������ɣ�
(4)ȡ��������������Һ���ȣ���Na2CO3��Һ���а�ɫ�������ɣ�
(5)��(3)�õ��ļ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������
��������ʵ����ʵȷ���� �ڸ���Һ�п϶����ڵ�������______________���϶������ڵ�������________________________________________________________________________
______________��������ȷ���Ƿ���ڵ�������________����μ��鲻��ȷ���������Ƿ���ڣ�________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com