
”¾ĢāÄæ”æ5ÖÖ¹ĢĢåĪļÖŹA”¢B”¢C”¢D”¢EÓÉĻĀ±ķÖŠ²»Ķ¬µÄŅõŃōĄė×Ó×é³É£¬ĖüĆĒ¾łŅ×ČÜÓŚĖ®”£

·Ö±šČ”ĖüĆĒµÄĖ®ČÜŅŗ½ųŠŠŹµŃ飬½į¹ūČēĻĀ£ŗ
¢ŁC£¬EČÜŅŗĻŌ¼īŠŌ£¬A£¬B£¬DČÜŅŗ³ŹĖįŠŌ£¬0.1mol£ÆLµÄEČÜŅŗpH£¼13£»
¢ŚBČÜŅŗÓėEČÜŅŗ»ģŗĻŗó²śÉśŗģŗÖÉ«³Įµķ£¬Ķ¬Ź±²śÉś“óĮæĘųĢ壻
¢ŪÉŁĮæCČÜŅŗÓėDČÜŅŗ»ģŗĻŗó²śÉś°×É«³Įµķ£¬¹żĮæCČÜŅŗÓėDČÜŅŗ»ģŗĻŗóĪŽĻÖĻó£»
¢Ü½«38.4 g CuʬĶ¶Čė×°ÓŠ×ćĮæDČÜŅŗµÄŹŌ¹ÜÖŠ£¬Cu²»Čܽā£¬ŌŁµĪ¼Ó1.6 mol”¤L£1Ļ”H2SO4£¬CuÖš½„Čܽā£¬¹ÜæŚø½½üÓŠŗģ×ŲÉ«ĘųĢå³öĻÖ”£
£Ø1£©¾Ż“ĖĶʶĻC”¢DµÄ»ÆѧŹ½ĪŖ£ŗC______________£»D_______________”£
£Ø2£©Š“³ö²½Öč¢ŚÖŠ·¢Éś·“Ó¦µÄĄė×Ó·“Ó¦·½³ĢŹ½____________________________”£
£Ø3£©²½Öč¢ÜÖŠČōŅŖ½«CuʬĶźČ«Čܽā£¬ÖĮÉŁ¼ÓČėĻ”H2SO4µÄĢå»żŹĒ____________mL”£
£Ø4£©²»ÄÜČ·¶ØµÄČÜŅŗĪŖBŗĶ______________(Ģī×ÖÄø±ąŗÅ£©”£
”¾“š°ø”æBa(OH£©2Al(NO3£©32Fe3++3CO32-+3H2O=2Fe(OH£©3”ż+3CO2”ü500A
”¾½āĪö”æ
¢ŁÓÉĢāÖŠŠÅĻ¢æÉÖŖ£¬C”¢EČÜŅŗĻŌ¼īŠŌ£¬ČÜŅŗæÉÄÜĪŖ¼īČÜŅŗ»ņĒæ¼īČõĖįŃĪ£¬A”¢B”¢DČÜŅŗ³ŹĖįŠŌ£»0.1mol£ÆLµÄEČÜŅŗpH£¼13£¬ŌņEÖŠČõĖįøłĄė×ÓĖ®½ā£¬øł¾ŻĄė×Ó¹²“ęæÉÖŖ£¬Eŗ¬ÓŠĢ¼ĖįøłĄė×Ó£¬½įŗĻĄė×Ó¹²“ę£¬EÖ»ÄÜĪŖĢ¼ĖįÄĘ£»½įŗĻĄė×Ó¹²“ęæÉÖŖ£¬CĪŖĒāŃõ»Æ±µ£»¢ŚBČÜŅŗÓėEČÜŅŗ»ģŗĻŗó²śÉśŗģŗÖÉ«³Įµķ£¬Ķ¬Ź±²śÉś“óĮæĘųĢ壬ŌņBÖŠŗ¬ÓŠĢśĄė×Ó£¬ĢśĄė×ÓÓėĢ¼ĖįøłĄė×Ó·¢ÉśĖ«Ė®½ā·“Ӧɜ³É¶žŃõ»ÆĢ¼ŗĶĒāŃõ»ÆĢśŗģŗÖÉ«³Įµķ£»¢ŪÉŁĮæCČÜŅŗÓėDČÜŅŗ»ģŗĻŗó²śÉś°×É«³Įµķ£¬¹żĮæĒāŃõ»ÆÄĘČÜŅŗÓėDČÜŅŗ»ģŗĻŗóĪŽĻÖĻó£¬ĖµĆ÷DÖŠŗ¬ÓŠĀĮĄė×Ó£¬ĒŅ²»ÄÜŹĒĮņĖįĀĮ£»¢Ü½«38.4 g CuʬĶ¶Čė×°ÓŠ×ćĮæDČÜŅŗµÄŹŌ¹ÜÖŠ£¬Cu²»Čܽā£¬ŌŁµĪ¼Ó1.6 mol”¤L£1Ļ”H2SO4£¬CuÖš½„Čܽā£¬¹ÜæŚø½½üÓŠŗģ×ŲÉ«ĘųĢå³öĻÖ£¬ĖµĆ÷DÖŠŗ¬ÓŠĻõĖįøłĄė×Ó£¬ŌņDĪŖĻõĖįĀĮ£¬ÄĒĆ“AĪŖĮņĖįĶ»ņÕßĀČ»ÆĶ£¬ŌņBĪŖĀČ»ÆĢś»ņĮņĖįĢś”£
(1)ÓÉŅŌÉĻ·ÖĪöæÉÖŖ£¬CĪŖBa(OH£©2£¬DĪŖAl(NO3£©3 £» (2) ²½Öč¢ŚĪŖĢ¼ĖįÄĘŗĶĢśĄė×ÓµÄĖ«Ė®½ā·“Ó¦£¬Ąė×Ó·½³ĢŹ½ĪŖ£ŗ2Fe3++3CO32-+3H2O=2Fe(OH£©3”ż+3CO2”ü£»(3)38.4æĖĶµÄĪļÖŹµÄĮæĪŖ38.4/64=0.6mol£¬²½Öč¢ÜÖŠ·¢ÉśµÄĄė×Ó·“Ó¦·½³ĢŹ½ĪŖ£ŗ3Cu+8H++2NO3-=3Cu2++2NO”ü+4H2O£¬ČōŅŖ½«ĶʬĶźČ«Čܽā£¬ŠčŅŖĒāĄė×ÓµÄĪļÖŹµÄĮæĪŖ1.6mol£¬¹ŹÖĮÉŁ¼ÓČėĻ”ĮņĖįµÄĢå»żÉčĪŖV£¬Ōņ1.6”ĮV”Į2=1.6£¬V=500mL£»ÓÉ·ÖĪöæÉÖŖAĪŖĮņĖįĶ»ņÕßĀČ»ÆĶ£¬ÄĒBĪŖĀČ»ÆĢś»ņĮņĖįĢś£¬AŗĶB¶¼²»ÄÜČ·¶Ø”£


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æijĶ¬Ń§ÓĆČēĶ¼ĖłŹ¾×°ĮæÖĘȔɣĮæ°Ä±½²¢Ö¤Ć÷øĆ·“Ó¦ŹĒČ”“ś·“Ó¦”£Ēė»Ų“š£ŗ

£Ø1£©ŅĒĘ÷AµÄĆū³ĘĪŖ____________£¬ĄäÄżĖ®µÄ½ųĖ®æŚĪŖ_________£¬(Ģī”°m”±»ņ”°n”±)”£
£Ø2£©ÖĘČ”äå±½µÄ»Æѧ·½³ĢŹ½ĪŖ___________________”£
£Ø3£©×¶ŠĪĘæÖŠ×ćĮæNaOHČÜŅŗµÄ×÷ÓĆŹĒ_________”£
£Ø4£©“ÓŹµŃé°²Č«µÄ½Ē¶Č·ÖĪö£¬øĆŹµŃé×°ÖĆ“ęŌŚŅ»“¦Ć÷ĻŌµÄȱĻŻĒėÖø³ö_________”£
£Ø5£©·“Ó¦½įŹųŗóĻņČż¾±ĘæÖŠµĪ¼ÓĒāŃõ»ÆÄĘČÜŅŗ£¬³ä·ÖÕńµ“£¬Č»ŗóÓĆ__________(ĢīŅĒĘ÷Ćū³Ę)·ÖĄė³öäå±½(ČŌŗ¬ÓŠÉŁĮæ±½)”£
£Ø6£©Éč¼ĘŹµŃéÖ¤Ć÷ÖĘČ”äå±½µÄ·“Ó¦ŹĒČ”“ś·“Ó¦___________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻÖÓŠČż×鏵Ńé£ŗ¢Ł³żČ„»ģŌŚÖ²ĪļÓĶÖŠµÄĖ®£»¢Ś»ŲŹÕµāµÄCCl4ČÜŅŗÖŠµÄCCl4£»¢ŪÓĆŹ³ÓĆ¾Ę¾«½žÅŻÖŠ²ŻŅ©ĢįČ”ĘäÖŠµÄÓŠŠ§³É·Ö”£ŅŌÉĻŹµŃéÕżČ··½·ØŅĄ“ĪŹĒ( )
A. ·ÖŅŗ”¢ŻĶČ””¢ÕōĮó B. ŻĶČ””¢ÕōĮ󔢷ÖŅŗ
C. ÕōĮó”¢ŻĶČ””¢·ÖŅŗ D. ·ÖŅŗ”¢ÕōĮó”¢ŻĶČ”
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÓŠ»śĪļHŹĒŅ»ÖÖÖŲŅŖµÄøß·Ö×Ó»ÆŗĻĪļ£¬ĘäŗĻ³ÉĀ·ĻßČēĻĀ£ŗ

ŅŃÖŖ£ŗ
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©AµÄĆū³ĘŹĒ_______________£¬CÖŠŗ¬Ńõ¹ŁÄÜĶÅĆū³ĘŹĒ______________”£
£Ø2£©Š“³ö·“Ó¦ĄąŠĶ£ŗA”śB______________£¬C”śD__________________”£
£Ø3£©B”śCµÄ·“Ó¦ŹŌ¼ĮŗĶ·“Ó¦Ģõ¼žŹĒ______________________”£
£Ø4£©D+E”śFµÄ·“Ó¦·½³ĢŹ½ŹĒ_________________”£
£Ø5£©GµÄ·Ö×ÓŹ½ŹĒ____________________”£
£Ø6£©Āś×ćĻĀĮŠĢõ¼žµÄFµÄĶ¬·ÖŅģ¹¹Ģå¹²ÓŠ__________ÖÖ(²»æ¼ĀĒĮ¢ĢåŅģ¹¹)”£
a.±½»·ÉĻÓŠĮ½øöČ”“ś»ł£¬ĪŽĘäĖū»·×“½į¹¹£»b.ŗ¬Ģ¼Ģ¼Čż¼ü£¬ĪŽ-C”ŌCOH½į¹¹”£
£Ø7£©¶ą»·»ÆŗĻĪļŹĒÓŠ»śŃŠ¾æµÄÖŲŅŖ·½Ļņ£¬ĒėÉč¼ĘÓÉ![]() ”¢.CH3CHO”¢
”¢.CH3CHO”¢![]() -CHOŗĻ³É¶ą»·»ÆŗĻĪļ
-CHOŗĻ³É¶ą»·»ÆŗĻĪļ µÄĀ·Ļß(ĪŽ»śŹŌ¼ĮČĪŃ”)______________
µÄĀ·Ļß(ĪŽ»śŹŌ¼ĮČĪŃ”)______________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÓĆĻĀĮŠŹµŃé×°ÖĆĶź³É¶ŌÓ¦µÄŹµŃé£Ø²æ·ÖŅĒĘ÷ĀŌČ„£©£¬ÄÜ“ļµ½ŹµŃéÄæµÄŹĒ£Ø””””£©
A.  ÖĘČ”ŅŅĖįŅŅõ„
ÖĘČ”ŅŅĖįŅŅõ„
B.  ĪüŹÕNH3
ĪüŹÕNH3
C.  ŹÆÓĶµÄ·ÖĮó
ŹÆÓĶµÄ·ÖĮó
D.  ±Č½ĻŃĪĖį”¢Ģ¼Ėį”¢±½·ÓµÄĖįŠŌĒæČõ
±Č½ĻŃĪĖį”¢Ģ¼Ėį”¢±½·ÓµÄĖįŠŌĒæČõ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æѧɜъ¾æŠŌѧĻ°Š”×éÉč¼ĘŹµŃ飬ÓĆÓŚÖĘČ”SO2²¢Ģ½¾æSO2µÄijŠ©ŠŌÖŹ”£ÖĘČ”SO2·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗNa2SO3+2H2SO4”śNa2SO4+SO2”ü+H2O£¬²śÉśµÄĘųĢåĶØČėČēĶ¼ĖłŹ¾×°ÖĆ£ŗ
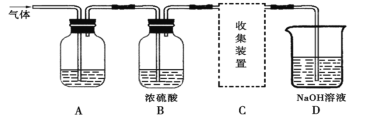
£ØŹµŃéĢ½¾æ£©
(1)½«12.6gµÄNa2SO3Óė×ćĮæµÄÅØĮņĖį·“Ó¦æÉÖʵĆSO2µÄĢå»żĪŖ___________L£Ø±ź×¼×“æö£©£¬øĆÖĘČ”SO2µÄ·“Ó¦________£ØŃ”Ģī”°ŹĒ”±»ņ”°²»ŹĒ”±£©Ńõ»Æ»¹Ō·“Ó¦”£
(2)ČōÓĆA×°ÖĆ¼ģŃéSO2¾ßÓŠĘư׊Ō£¬ŌņAÖŠµÄČÜŅŗŹĒ_________”£
ČōÓĆA×°ÖĆ¼ģŃéSO2ŹĒŅ»ÖÖĖįŠŌŃõ»ÆĪļ£¬ŌņAÖŠµÄČÜŅŗŹĒ_________”£
(3)D×°ÖĆÖŠ·¢ÉśµÄ·“Ó¦ŹĒ£ØŠ“»Æѧ·½³ĢŹ½£©£ŗ____________________________________”£
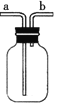
£ØŹµŃéĢÖĀŪ£©
(4)¶ŌČēĶ¼ÖŠµÄC“¦£¬¼×”¢ŅŅĮ½Ķ¬Ń§¶¼Ń”ÓĆČēĶ¼×°ÖĆ£¬µ«¶ŌĮ¬½Ó·½Ź½³ÖÓŠ²»Ķ¬Ņā¼ū”£¼×Ķ¬Ń§ČĻĪŖ£ŗSO2ĘųĢåÓ¦“Óa“¦ĶØČė¼ÆĘųĘæÖŠ”£ŅŅĶ¬Ń§ČĻĪŖ£ŗSO2ĘųĢåÓ¦“Ób“¦ĶØČė¼ÆĘųĘæÖŠ”£ÄćČĻĪŖ________£ØĢī”°¼×”±»ņ”°ŅŅ”±£©Ķ¬Ń§µÄæ“·ØŹĒÕżČ·µÄ”£
£ØĮŖĻµŹµ¼Ź£©
(5)SO2¶Ō»·¾³µÄÓ°Ļģ½Ļ“ó£¬ĪŖĮĖ¼õÉŁSO2¶ŌæÕĘųµÄĪŪČ¾£¬ĒėÄć“Ó¹¤ŅµÉś²śµÄ½Ē¶ČĢį³öŅ»ÖÖÓŠŠ§æÉŠŠµÄ“ėŹ©£ØÓĆĪÄ×Ö±ķŹö£©£ŗ________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æß»ą«·ÓŹĒŗĻ³ÉÅ©Ņ©µÄÖŲŅŖÖŠ¼äĢ壬ĘäŗĻ³ÉĀ·ĻßČēĻĀ£ŗ
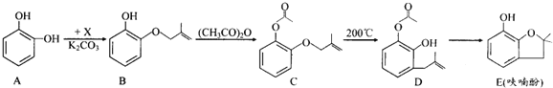
£Ø1£©AŌŚæÕĘųÖŠ¾ĆÖĆ»įÓÉĪŽÉ«×Ŗ±äĪŖ×ŲŗÖÉ«£¬ĘäŌŅņŹĒ____________£¬AŌŚŗĖ“Ź²ÕńĒāĘ×ÖŠÓŠ___________×é·å”£
£Ø2£©B”śCµÄ·“Ó¦ĄąŠĶŹĒ_____________________”£
£Ø3£©ŅŃÖŖXµÄ·Ö×ÓŹ½ĪŖC4H7Cl”£Š“³öA”śBµÄ»Æѧ·½³ĢŹ½£ŗ___________________”£
£Ø4£©ŅŖ¼ų±š»ÆŗĻĪļCŗĶD£¬ŹŹŅĖµÄŹŌ¼ĮŹĒ__________________________”£
£Ø5£©BµÄĶ¬·ÖŅģ¹¹Ģåŗܶą£¬·ūŗĻĻĀĮŠĢõ¼žµÄÓŠ______ÖÖ£¬Š“³öĘäÖŠÄÜ·¢ÉśŅų¾µ·“Ó¦µÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½£ŗ__________(ČĪŠ“Ņ»ÖÖ)”£
¢Ł±½µÄŃÜÉśĪļ””¢ŚÓŠĮ½øö»„ĪŖ¶ŌĪ»µÄČ”“ś»ł ¢Ūŗ¬ÓŠõ„»ł
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĄūÓĆĖį½ā·ØÖĘīŃ°×·Ū²śÉśµÄ·ĻŅŗ[ŗ¬ÓŠ“óĮæFeSO4”¢H2SO4ŗĶÉŁĮæFe2(SO4)3”¢TiOSO4]£¬Éś²śĢśŗģŗĶ²¹ŃŖ¼ĮČéĖįŃĒĢś”£ĘäÉś²ś²½ÖčČēĻĀ£ŗ

ŅŃÖŖ£ŗTiOSO4æÉČÜÓŚĖ®£¬ŌŚĖ®ÖŠæÉŅŌµēĄėĪŖTiO2+ŗĶSO42-£¬TiOSO4Ė®½ā³ÉTiO2xH2O³ĮµķĪŖæÉÄę·“Ó¦£»ČéĖį½į¹¹¼ņŹ½ĪŖCH3CH(OH)COOH”£
Ēė»Ų“š£ŗ
£Ø1£©²½Öč¢ŁÖŠ·ÖĄėĮņĖįŃĒĢśČÜŅŗŗĶĀĖŌüµÄ²Ł×÷ŹĒ________________________”£
£Ø2£©¼ÓČėĢśŠ¼µÄÄæµÄŅ»ŹĒ»¹ŌÉŁĮæFe2(SO4)3£»¶žŹĒŹ¹ÉŁĮæTiOSO4×Ŗ»ÆĪŖTiO2xH2OĀĖŌü£¬ÓĆĘ½ŗāŅĘ¶ÆµÄŌĄķ½āŹĶµĆµ½ĀĖŌüµÄŌŅņ___________________________”£
£Ø3£©ĮņĖįŃĒĢśŌŚæÕĘųÖŠģŃÉÕÉś³ÉĢśŗģŗĶČżŃõ»ÆĮņ£¬øĆ·“Ó¦ÖŠŃõ»Æ¼ĮŗĶ»¹Ō¼ĮµÄĪļÖŹµÄĮæÖ®±ČĪŖ____________________________”£
£Ø4£©ÓĆĄė×Ó·½³ĢŹ½½āŹĶ²½Öč¢ŻÖŠ¼ÓČéĖįÄܵƵ½ČéĖįŃĒĢśµÄŌŅņ_________________”£
£Ø5£©²½Öč¢ÜµÄĄė×Ó·½³ĢŹ½ŹĒ_________________________________________”£
£Ø6£©²½Öč¢Ž±ŲŠėæŲÖĘŅ»¶ØµÄÕęæÕ¶Č£¬ŌŅņŹĒÓŠĄūÓŚÕō·¢Ė®ŅŌ¼°___________________”£
£Ø7£©ĪŖ²ā¶Ø²½Öč¢ŚÖŠĖłµĆ¾§ĢåÖŠFeSO4”¤7H2OµÄÖŹĮæ·ÖŹż£¬Č”¾§Ģåѳʷa g£¬ČÜÓŚĻ”ĮņĖįÅä³É100.00 mLČÜŅŗ£¬Č”³ö20.00 mLČÜŅŗ£¬ÓĆKMnO4ČÜŅŗµĪ¶Ø£ØŌÓÖŹÓėKMnO4²»·“Ó¦£©”£ČōĻūŗÄ0.1000 molL-1 KMnO4ČÜŅŗ20.00 mL£¬ĖłµĆ¾§ĢåÖŠFeSO4”¤7H2OµÄÖŹĮæ·ÖŹżĪŖ______£ØÓĆa±ķŹ¾£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”湤ŅµÉĻŅŌCOĪŖŌĮĻÉś²ś¶ž¼×Ćѵķ“Ó¦ĪŖ£ŗ3H2(g)£«3CO(g) ![]() CH3OCH3(g)£«CO2(g) ”÷H£½a kJmol-1 T”ꏱ£¬ĘšŹ¼Ź±ŌŚŗćČŻĆܱÕČŻĘ÷ÖŠ¼ÓČėŅ»¶ØĮæµÄH2ŗĶCO£¬ŹµŃéÄŚČŻŗĶ½į¹ūČēĻĀ±ķŗĶĻĀĶ¼ĖłŹ¾”£
CH3OCH3(g)£«CO2(g) ”÷H£½a kJmol-1 T”ꏱ£¬ĘšŹ¼Ź±ŌŚŗćČŻĆܱÕČŻĘ÷ÖŠ¼ÓČėŅ»¶ØĮæµÄH2ŗĶCO£¬ŹµŃéÄŚČŻŗĶ½į¹ūČēĻĀ±ķŗĶĻĀĶ¼ĖłŹ¾”£
ŹµŃé ŠņŗÅ | ČŻĘ÷ Ģå»ż | ĘšŹ¼ĪļÖŹµÄĮæ | “ļĘ½ŗāŹ± ·Å³öČČĮæ | |
H2 | CO | |||
¢ń | 2L | 8mol | 8mol | 494 kJ |
¢ņ | 2L | 4mol | 4mol | ”Ŗ”Ŗ |

£Ø1£©ÉĻŹö·“Ó¦Ę½ŗā³£ŹżKµÄ±ķ“ļŹ½ĪŖ_____”£
£Ø2£©ÓÉĢāŅāæÉÖŖ£¬a£½______£¬b________1(Ģī”°£¾”±”¢”°£¼”±»ņ”°£½”±)”£
£Ø3£©ŹµŃé¢ńÖŠ£¬·“Ó¦Ē°10 minÄŚµÄĘ½¾łĖŁĀŹv(H2)£½_____”£
£Ø4£©ĻĀĮŠĢõ¼žÄÜŹ¹ÉĻŹö·“Ó¦µÄ·“Ó¦ĖŁĀŹŌö“ó£¬ĒŅĘ½ŗāĻņÕż·“Ó¦·½ĻņŅĘ¶ÆµÄŹĒ______ (ĢīŠ“ŠņŗÅ×ÖÄø)”£
a£®¼°Ź±·ÖĄė³öCH3OCH3ĘųĢåb£®±£³ÖČŻĘ÷µÄČŻ»ż²»±ä£¬ŌŁ³äČė1 mol COŗĶ1 mol H2
c£®ŹŹµ±ÉżøßĪĀ¶Č d£®±£³ÖČŻĘ÷µÄČŻ»ż²»±ä£¬³äČė1 mol ŗ¤Ęų
£Ø5£©T”ꏱ£¬ČōČŻĘ÷ÖŠŗ¬1 molL-1 H2”¢2 molL-1 CO”¢2 molL-1 CH3OCH3”¢3 molL-1 CO2£¬Ōņ“ĖŹ±vÕż________vÄę(Ģī”°£¾”±”¢”°£¼”±»ņ”°£½”±)”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com