
���ǵؿ��к�����ߵĽ���Ԫ�أ��䵥�ʼ��Ͻ������������е�Ӧ�������㷺��
���̼�Ȼ�ԭ���Ȼ�����ʵ�����������Ʊ�������������ط�Ӧ���Ȼ�ѧ����ʽ���£�
Al2O3(s)��AlCl3(g)��3C(s)=3AlCl(g)��3CO(g)
��H��a kJ��mol��1
3AlCl(g)=2Al(l)��AlCl3(g)��H��b kJ��mol��1
(1)��ӦAl2O3(s)��3C(s)=2Al(l)��3CO(g)�Ħ�H��________kJ��mol��1(�ú�a��b�Ĵ���ʽ��ʾ)��
(2)Al4C3�Ƿ�Ӧ�����е��м���Al4C3�����ᷴӦ(����֮һ�Ǻ�������ߵ���)�Ļ�ѧ����ʽΪ______________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
2013��12��15��4ʱ���س���ϵ�л���ġ����úš�˳��ʻ��������棬ʵ�������Ǻ���ҫ����Ĵ��١����������Ҫ����ȼ�ϣ�ͨ�����£�N2H4����ȼ�ϣ�N2O4������������ش��������⣺
��1����֪��N2(g) + 2O2(g) ="=" 2NO2(g) ��H= + 67.7kJ��mol-1
N2H4(g) + O2��g��="=" N2(g) + 2H2O(g) ��H= - 534.0kJ��mol-1
2NO2(g)  N2O4��g�� ��H=" -" 52.7kJ��mol-1
N2O4��g�� ��H=" -" 52.7kJ��mol-1
д����̬������̬������������ȼ�����ɵ�������̬ˮ���Ȼ�ѧ����ʽ�� ��
��2����ҵ���ô�������������İ�����Ӧ�Ʊ��£��÷�Ӧ�Ļ�ѧ����ʽΪ�� ��
��3����ҵ�Ͽ��������з�Ӧԭ���Ʊ�������
2N2(g)+6H2O(l) 4NH3(g)+3O2(g) ��H= Q kJ��mol-1
4NH3(g)+3O2(g) ��H= Q kJ��mol-1
����֪�÷�Ӧ��ƽ�ⳣ��K���¶ȵĹ�ϵ��ͼ����˷�Ӧ�� Q 0 ���������������=������
������ʼ���뵪����ˮ��15���Ӻ�Ӧ�ﵽƽ�⣬��ʱNH3��Ũ��Ϊ0.3mol/L������������ʾ�ķ�Ӧ����Ϊ ��
�۳����£����������Ӧ�����������ܱ������з���������Ӧ�ﵽƽ��ʱ�� ��ѡ���ţ�.
| A�������������ƽ����Է�����������ʱ����仯 |
| B��v��N2��/v��O2��=2��3 |
| C��������������ܶȲ���ʱ����仯 |
| D��ͨ��ϡ����������߷�Ӧ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���Ŀǰ���緶Χ�ڵ���ԴΣ�����״���Ϊһ�ֽϺõĿ�������Դ�����й㷺��Ӧ��ǰ����
��1����֪�ڳ��³�ѹ�·�Ӧ���Ȼ�ѧ����ʽ��
��CO��g����2H2��g��  CH3OH��g������H1����90 kJ��mol��1
CH3OH��g������H1����90 kJ��mol��1
��CO��g����H2O��g��  CO2��g����H2��g����H2����41 kJ��mol��1
CO2��g����H2��g����H2����41 kJ��mol��1
д���ɶ�����̼�������Ʊ��״����Ȼ�ѧ����ʽ��_______________________��
��2�����ݻ�ΪV L�������г���a mol CO��2a mol H2���ڴ��������·�Ӧ���ɼ״���ƽ��ʱ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
��p1________p2������ڡ�����С�ڡ����ڡ�����
���������������������£�������a mol CO��2a mol H2���ﵽ��ƽ��ʱ��CO��ת����________�����������С�����䡱����ͬ����ƽ�ⳣ��________��
��3����֪��T ��ʱ��CO��g����H2O��g��??CO2��g����H2��g����ƽ�ⳣ��K��0.32���ڸ��¶��£���֪cʼ��CO����1 mol��L��1��cʼ��H2O����1 mol��L��1��ijʱ�̾��ⶨCO��ת����Ϊ10%����÷�Ӧ________����Ѿ�����û�С����ﵽƽ�⣬ԭ����___________________________________________����ʱ��v��________v�����>����<������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ����
��1����ҵ��һ������������ַ�Ӧ�ϳɼ״���
��Ӧ��CO��g����2H2��g��=CH3OH��g������H1
��Ӧ��CO2��g����3H2��g��=CH3OH��g����H2O��g������H2
��������Ӧ���ϡ�ԭ�Ӿ��á�ԭ�����________�������
���±����������Ƿ�Ӧ���ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ����K����
| �¶� | 250 �� | 300 �� | 350 �� |
| K | 2.041 | 0.270 | 0.012 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)�ڱ���¯�з�����Ӧ��
��Fe2O3(s)��3C(s) 2Fe(s)��3CO(g)����H����492.7 kJ��mol��1
2Fe(s)��3CO(g)����H����492.7 kJ��mol��1
��3CO(g)��Fe2O3(s)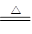 2Fe(s)��3CO2(g)����H����25.2 kJ��mol��1
2Fe(s)��3CO2(g)����H����25.2 kJ��mol��1
��Ӧ2Fe2O3(s)��3C(s)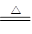 4Fe(s)��3CO2(g)����H��________kJ��mol��1��
4Fe(s)��3CO2(g)����H��________kJ��mol��1��
(2)��Ȼ��(�Լ����)�ڹ�ҵ��������;�㷺����������ת������H2����Ҫת����Ӧ���£�
CH4 (g)��H2O(g) CO(g)��3H2(g)����H����206.2 kJ��mol��1
CO(g)��3H2(g)����H����206.2 kJ��mol��1
CH4(g)��2H2O(g) CO2(g)��4H2(g)����H����165.0 kJ��mol��1
CO2(g)��4H2(g)����H����165.0 kJ��mol��1
������Ӧ����ԭ�����е�CO��ʹ���ϳɴ����ж��������ȥ����ҵ�ϳ����ô���������CO��ˮ������Ӧ�����׳�ȥ��CO2��ͬʱ�ֿ��Ƶõ�����������ķ������˷�Ӧ��Ϊһ����̼�任��Ӧ���÷�Ӧ���Ȼ�ѧ����ʽ��_________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������繤ҵ���õķ�չ���˿ڵľ�����ȫ����Դ���ż�������������Խ��Խ���ص����⣬��ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2������ȫ������ձ����ӡ�
��1����ͼΪC����������ı仯��ϵͼ�����ٱ仯���û���Ӧ�����仯ѧ����ʽ��Ϊ______________________________________��
ͼ�б仯������Щ�����ȷ�Ӧ________������ţ���
��2���״���һ�ֿ�������Դ�����п�����Ӧ�õĹ���ǰ������ҵ�Ͽ������·����ϳɼ״���
����һ��CO��g����2H2��g��??CH3OH��g��
��������CO2��g����3H2��g��??CH3OH��g����H2O��g��
��25�桢101 kPa�£�1�˼״���ȫȼ�շ���22.68 kJ��д���״�ȼ�յ��Ȼ�ѧ����ʽ��_____________________________________________��
ij�������糧CO2������ŷ�����2 200��֣�������CO2��ȫת��Ϊ�״������������ɴ˻�õļ״���ȫȼ�շ���Լ��________kJ��������λ��Ч���֣���
��3��������ұ������������һ����Ӧ�ǽ�ԭ�Ͻ��ʯת����TiO2�����ʯ����2C��2Cl2����,TiCl4��2CO����֪��C��s����O2��g��=CO2��g������H����393.5 kJ��mol��1
2CO��g����O2��g��=2CO2��g������H����566 kJ��mol��1
TiO2��s����2Cl2��g��=TiCl4��s����O2��g������H����141 kJ��mol��1
��TiO2��s����2Cl2��g����2C��s��=TiCl4��s����2CO��g���Ħ�H��________��
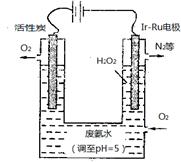
��4�����������ھ�������������ˮ������������ҵ�������ΪƯ�����������������������ҿ������������е��ʷ�Ӧ���磺
6Ag��s����O3��g��=3Ag2O��s������H����235.8 kJ��mol��1��
��֪��2Ag2O��s��=4Ag��s����O2��g����H����62.2 kJ��mol��1��
��O3ת��ΪO2���Ȼ�ѧ����ʽΪ_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�״���һ����Ҫ�Ļ���ԭ�ϡ��״���ˮ�����������ɻ�������Դ�����й㷺��Ӧ��ǰ������������ʵ�飬�����Ϊ1 L���ܱ������У�����1mol CH3OH��1molH2O��һ�������·�����Ӧ��CH3OH (g)+ H2O (g) CO2(g) +3 H2 (g)�����CO2��CH3OH(g)��Ũ����ʱ��仯���±���ʾ��
CO2(g) +3 H2 (g)�����CO2��CH3OH(g)��Ũ����ʱ��仯���±���ʾ��
| ʱ�� ���� | 0 min | 10 min | 30 min | 60 min | 70 min |
| CO2(mol/L) | 0 | 0.2 | 0.6 | 0.8 | 0.8 |
| CH3OH(mol/L) | 1.0 | 0.8 | 0.4 | 0.2 | 0.2 |
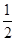 O2 (g)
O2 (g) CO2(g) + 2H2 (g) ?H1= ��192.9kJ/mol
CO2(g) + 2H2 (g) ?H1= ��192.9kJ/mol 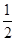 O2 (g)
O2 (g) H2 O(g) ?H2= ��120.9kJ/mol
H2 O(g) ?H2= ��120.9kJ/mol 
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������ѳ�Ϊ��ǰ��δ����һ��ȫ�����ش���⡣Ϊ���Ŀǰȼ��ʹ�ù����еĻ�����Ⱦ���⣬��������ԴΣ�����е�ר���������̫���ܴ�ʹȼ��ѭ��ʹ�õĹ��룬��ͼ��ʾ��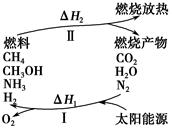
���̢�������·�Ӧ��ʾ��
��2CO2 2CO��O2����2H2O===2H2��O2����2N2��6H2O
2CO��O2����2H2O===2H2��O2����2N2��6H2O 4NH3��3O2����2CO2��4H2O
4NH3��3O2����2CO2��4H2O 2CH3OH��3O2����2CO��4H2O
2CH3OH��3O2����2CO��4H2O ________��3O2
________��3O2
��ش��������⣺
(1)���̢������ת����ʽΪ________��ת��Ϊ________�ܡ�
(2)����ɵڢݸ���Ӧ�Ļ�ѧ����ʽ��____________________��
(3)����ת�������У���H1�ͦ�H2�Ĺ�ϵ��________��
(4)����1 mol��ѧ��������������±���
| ���ۼ� | H��N | H��O | N��N | O===O |
| ����1 mol��ѧ����������/(kJ��mol��1) | 393 | 460 | 941 | 499 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����Ź㷺����;�������ڻ��ʡ����ᡢ�ϳ���ά�ȹ�ҵ������
��1���������¡��˹��̵������о��������ڳ��¡���ѹ�����������£�N2�ڴ�������������Fe2O3��TiO2��������ˮ������Ӧ�����ɰ�����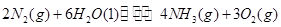
�÷�Ӧ�ڹ̶�������ܱ������н��У��й�˵����ȷ����_____________���������ĸ����
A����Ӧ����ƽ��״̬ʱ��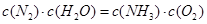 |
B����Ӧ�ﵽƽ���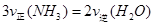 |
| C����ϵ����ѹǿ���䣬˵����Ӧ�Ѵ�ƽ�� |
| D�����������ܶȱ��ֲ��䣬˵����Ӧ�Ѵ�ƽ�� |
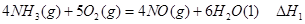 ��
��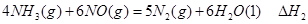 ��
��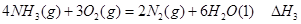 ��
��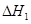 ��
��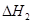 ��
��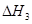 ����֮���ϵ�ı���ʽ��
����֮���ϵ�ı���ʽ��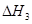 ��_________��
��_________��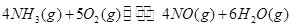
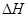 ��
��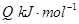


| ʱ��/Ũ�� |  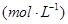 | 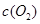 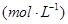 | 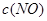 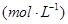 | 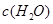 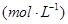 |
| ��ʼ | 4.0 | 5.5 | 0 | 0 |
| ��2min | 3.2 | a | 0.8 | 1.2 |
| ��4min | 2.0 | 3.0 | 2.0 | 3.0 |
| ��6min | 2.0 | 3.0 | 2.0 | 3.0 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com