
��ͼ����ʾʵ��װ��֤������ͭ�ܼӿ�Լ7%��˫��ˮ�ķֽⲢ��������̵Ĵ�Ч�����бȽ�(���ȽϷ�Ӧ����)����ͼʾװ�ò�������������������������Ӱ��ʵ������ؾ��Ѻ��ԣ�����������£�

(1)��ʵ��ԭ��������ʵ���еġ��������ݡ�����ָ ��Ҳ����ָ ��
(2)ʵ��ʱ�����ռ���B�У�B����������__________����Ҫ���������������O2���������ռ��������õ��ɼм�סB�¶��齺�ܣ�������Ƥ���� ��

(3)Ϊ̽��CuO��ʵ������Ƿ�������ã�����ٱȽ��⣬���貹������ʵ�鲻��д���岽��)��a��֤��CuO�Ļ�ѧ����û�б仯��b�� ��
(4)Ϊ֤������ͭ�Ļ�ѧ�����ڼ���˫��ˮǰ��û�з����ı䣬�������֤��ʵ���� ��
(5)ʵ�鿪ʼʱ,���������м���һ������˫��ˮ��,���ڶ�ʱ���ڲ�����������,��Һ©���ڵ�Һ�岻��˳������,Ϊ�˽���������,���ȡ�Ĵ�ʩ��
;
�ڲ������ɵ��������ʱ,����Ҫע�������밼Һ����ƽ����,��Ӧע��
��1��ʱ��������������ͬʱ���ڷų�������ռ���������һ��ʱ�����Ҳ�ˮ�������ĸ߶�)��Ҳ����ָ������ͬ�����������Ҫ��ʱ��
��2������ܣ��Ѵ����ǵ�ľ���������ܣ����Ƿ�ȼ
��3��֤��CuO�������ڷ�Ӧǰ��û�б仯
��4���ֱ�ȡ����˫��ˮǰ�������ͭ��������H2����CO��C�Ȼ�ԭ��)�ķ�Ӧ��������Һ�ķ�Ӧ������ʵ��Ƚ�
��5��ʹ�����ϵİ��۶�ƿ����С�ף���������Һ����ƽ����Ӧǰ�������¶���ͬ
��������
�����������1��ʵ��Ŀ���DZȽϷ�Ӧ���ʣ�����ʵ���еġ��������ݡ�����ָʱ��������������ͬʱ���ڷų�������ռ���������һ��ʱ�����Ҳ�ˮ�������ĸ߶�)��Ҳ����ָ������ͬ�����������Ҫ��ʱ�䡣
��2�����������Ĺ����ص��֪��B���������Ǹ���ܡ���������ȼ�����壬���Լ���������ʵ�鷽���ǰѴ����ǵ�ľ���������ܣ����Ƿ�ȼ��
��3���ж�һ�������Ǵ��������ܸı䷴Ӧ�ٶ��⣬��Ҫ��֤��һ���������䣬�����仯ѧ���ʲ��䣬��֤��CuO�������ڷ�Ӧǰ��û�б仯��
��4����������ͭ�Ļ�ѧ���ʿ�֪��Ҫ֤������ͭ�Ļ�ѧ�����ڼ���˫��ˮǰ��û�з����ı䣬�����֤��ʵ������Ƿֱ�ȡ����˫��ˮǰ�������ͭ��������H2����CO��C�Ȼ�ԭ��)�ķ�Ӧ��������Һ�ķ�Ӧ������ʵ��Ƚϡ�
��5��ҪʹҺ��˳��������Ӧ��������ѹǿ��ͬ�����Բ�ȡ�Ĵ�ʩ��ʹ�����ϵİ��۶�ƿ����С�ף��ڲ����������ʱ��Ӧ���������ѹǿ��ͬ�����¶�Ҳ����ͬ�����Դ�ʩ��ʹ��������Һ����ƽ���ҷ�Ӧǰ�������¶���ͬ��
���㣺���鷴Ӧ���ʲⶨʵ���̽����������ʶ�������ļ��顢��������֤�Լ�����ʵ�����
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������ǿ����ע�ضԻ���֪ʶ���̺�ѵ����ͬʱ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ���淶�Ͻ���ʵ����������Լ���������������������Ҫ���Գ���������ѡ�á�ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ�Լ��������֪ʶ���ʵ�������������


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012���˽̰���л�ѧѡ��6 3.1 ���ʵļ�����ϰ���������棩 ���ͣ������

ijͬѧ����1���ȶ����ˮ��ʵ�飬���������е���Ԫ�ء�ʵ��������£�
��ͼ��ʾ���ڴ��Թ��м���5 mL 1 mol��L��1NaOH��Һ��5 mL 1?�ȶ���(1?�ȶ���ķе�Ϊ77��78 �棬�ܶ�Ϊ0.886 g��cm��3����ȼ)��ˮԡ���ȸ��Թ�10 min���ϣ������Ƽ����¶���70��80 �档ȡ2֧С�Թܣ�������Լ1 mL��2%��AgNO3��3 mol��L��1���ᰴ1��1������ɵĻ����Һ���ý�ͷ�ι���ȡ���Ⱥ���Թ��ڵ��ϲ���Һ�����˴���Һ��μ�������һ֧С�Թ��У�����һ֧δ�Ӵ���Һ��С�Թ�����Һ��ȣ��а�ɫ�Ļ�������֣�˵��1���ȶ�����NaOH��Һ��Ӧ��Cl�����ɣ��Ӷ�֤����1?�ȶ����к�����Ԫ�ء�
��ش�
(1)��д��1?�ȶ���ˮ��Ļ�ѧ����ʽ��
________________________________________________________________________��
(2)�Թ��Ϸ��IJ������ܵ�����Ϊ__________________��
(3)����Cl��ʱ���������ữAgNO3��Һ��Ŀ����
________________________________________________________________________��
(4)��װ�����л���ѧʵ���г��õ�һ��װ�ã�ʵ�����У�����ʹ������װ���Ʊ�����________(�����)��
A���������������������������� B����ϩ
C���������� D���屽
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ڳ���Ԫ���γɵĴ�����A��B��C��D��Eת����ϵ����ͼ��ʾ������A������B֮˾�ķ�Ӧ������Һ�н���(E������A��B���������е�ij����ͬ)
 |
��ͬ���������⣺
(1)��C�����ӻ����D��һ��ǿ�д��C�Ļ�ѧʽ ��
(2)��C��ˮ�������ֽⷴӦ��E��ˮ��Һ���������ԣ�D�Ǽ�������ǿ�ᡢ����
����ǿ��Ļ����д��ʵ������ȡD�����ӷ���ʽΪ ��
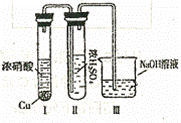 (3)��C��һ�����壬D��һ��ǿ�ᣬ��:
(3)��C��һ�����壬D��һ��ǿ�ᣬ��:
��C��ˮ��Ӧ�Ļ�ѧ����ʽΪ ��
��������Ϊ��ŨH2SO4���Ը�������C����ijͬѧΪ����
֤�ù۵��Ƿ���ȷ������ͼװ�ý���ʵ�顣
ʵ������У�ŨH2SO4��Ϊ����ɫ���Ң�װ��ĩ������������
�������������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com