
A”¢B¶¼ŹĒ·¼Ļć×å»ÆŗĻĪļ£¬1 mol AĖ®½āµĆµ½1 mol BŗĶ1 mol“×Ėį”£A”¢BµÄĻą¶Ō·Ö×ÓÖŹĮ涼²»³¬¹ż200£¬ĶźČ«Č¼ÉÕ¶¼Ö»Éś³ÉCO2ŗĶH2O”£ĒŅB·Ö×ÓÖŠCŗĶHŌŖĖŲ×ܵÄÖŹĮæ°Ł·Öŗ¬ĮæĪŖ65.2%(¼“ÖŹĮæ·ÖŹżĪŖ0.625)”£AČÜŅŗ¾ßÓŠĖįŠŌ£¬²»ÄÜŹ¹FeCl3(aq)ĻŌÉ«”£
(1)Mr(A)£Mr(B)= ;
(2)1øöB·Ö×ÓÖŠµÄOŌ×ÓøöŹżŹĒ______£»
(3)AµÄ·Ö×ÓŹ½ŹĒ______£»
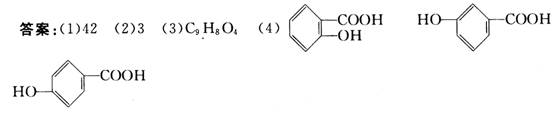
(4)BæÉÄܵÄČżÖÖ½į¹¹¼ņŹ½·Ö±šŹĒ______”¢______”¢______”£
ÓÉĢāŅāÖŖ£ŗA£«H2O![]() CH3COOH£«B£¬øł¾ŻÖŹĮæŹŲŗć¶ØĀÉµĆ£ŗMr(A)£«18=60£«Mr(B)£¬Mr(A)£Mr(B)=60£18=42”£Ōņ£ŗMr(B)=Mr(A)£42£¼200£42=158”£
CH3COOH£«B£¬øł¾ŻÖŹĮæŹŲŗć¶ØĀÉµĆ£ŗMr(A)£«18=60£«Mr(B)£¬Mr(A)£Mr(B)=60£18=42”£Ōņ£ŗMr(B)=Mr(A)£42£¼200£42=158”£
ÓÉ”°AČÜŅŗ¾ßÓŠĖįŠŌ£¬²»ÄÜŹ¹FeCl3(aq)ĻŌÉ«”±ÖŖ£¬A·Ö×ÓÖŠÓŠōČ»ł£¬ÄĒĆ“B·Ö×ÓÖŠŅ²ÓŠōČ»ł£¬ĒŅÓŠ“ÓA(“×Ėįijõ„)Ė®½ā³öµÄōĒ»ł£¬ÕāŃł£¬B·Ö×ÓÖŠÖĮÉŁÓŠ3øöŃõŌ×Ó”£ČōB·Ö×ÓÖŠŗ¬ÓŠ4øöŃõŌ×Ó£¬ŌņMr(B)=![]() =184£¬ÓėMr(B)£¼158Ƭ¶Ü£¬ÓŚŹĒČ·ČĻB·Ö×ÓÖŠŗ¬ÓŠ3øöŃõŌ×Ó”£Ōņ£ŗMr(B)=
=184£¬ÓėMr(B)£¼158Ƭ¶Ü£¬ÓŚŹĒČ·ČĻB·Ö×ÓÖŠŗ¬ÓŠ3øöŃõŌ×Ó”£Ōņ£ŗMr(B)= ![]() =138£¬½įŗĻBĪŖ·¼Ļć×å»ÆŗĻĪļĶĘÖŖ£ŗBĪŖōĒ»ł¼×Ėį(C7H6O3)£¬ŌņA·Ö×ÓŹ½ĪŖ£ŗC9H8O4”£
=138£¬½įŗĻBĪŖ·¼Ļć×å»ÆŗĻĪļĶĘÖŖ£ŗBĪŖōĒ»ł¼×Ėį(C7H6O3)£¬ŌņA·Ö×ÓŹ½ĪŖ£ŗC9H8O4”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 Ӣ
Ӣ Ӣ
”¢ £ØČĪŠ“Į½ÖÖ£©
£ØČĪŠ“Į½ÖÖ£© ”¢
Ӣ Ӣ
”¢ £ØČĪŠ“Į½ÖÖ£©
£ØČĪŠ“Į½ÖÖ£©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ






²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 Ӣ
Ӣ Ӣ
Ӣ
 Ӣ
Ӣ Ӣ
Ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø6·Ö£©A”¢B¶¼ŹĒ·¼Ļć×å»ÆŗĻĪļ£¬1 mol AĖ®½āµĆµ½1 mol BŗĶ1 mol“×Ėį”£A”¢BµÄĻą¶Ō·Ö×ÓÖŹĮ涼²»³¬¹ż200£¬ĶźČ«Č¼ÉÕ¶¼Ö»Éś³ÉCO2ŗĶH2O”£ĒŅB·Ö×ÓÖŠCŗĶHŌŖĖŲ×ܵÄÖŹĮæ°Ł·Öŗ¬ĮæĪŖ65.2%(¼“ÖŹĮæ·ÖŹżĪŖ0.625)”£AČÜŅŗ¾ßÓŠĖįŠŌ£¬²»ÄÜŹ¹FeCl3(aq)ĻŌÉ«”£
(1)Mr(A)£Mr(B)= ;
(2)1øöB·Ö×ÓÖŠµÄOŌ×ÓøöŹżŹĒ £»
(3)AµÄ·Ö×ÓŹ½ŹĒ £»
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com