
ŅŃÖŖ2SO2(g)£«O2(g)2SO3(g)””¦¤H<0µÄŹµŃ鏿¾ŻČēĻĀ±ķ£ŗ
| ĪĀ¶Č | ²»Ķ¬Ń¹ĒæĻĀSO2µÄ×Ŗ»ÆĀŹ(%) | ||||
| 1”Į105Pa | 5”Į105Pa | 1”Į106Pa | 5”Į106Pa | 1”Į107Pa | |
| 450”ę | 97.5 | 98.9 | 99.2 | 99.6 | 99.7 |
| 550”ę | 85.6 | 92.9 | 94.9 | 97.7 | 98.3 |
(1)ӦєŌńµÄĪĀ¶ČŹĒ________”£
(2)Ó¦²ÉÓƵÄŃ¹ĒæŹĒ________£¬ĄķÓÉŹĒ
________________________________________________________________________________________________________________________________________________ӣ
(3)ŌŚŗĻ³ÉSO3µÄ¹ż³ĢÖŠ£¬²»ŠčŅŖ·ÖĄė³öSO3µÄŌŅņŹĒ________________________________________________________________________________________________________________________________________________________________________________________________________________________”£
½āĪö£ŗøĆ·“Ó¦ÓėŗĻ³É°±µÄ·“Ó¦ĻąĖĘ£ŗ¶¼ŹĒĘųĢåĢå»żĖõŠ”µÄ·ÅČČ·“Ó¦£¬ĪĀ¶ČÉżøßÓŠĄūÓŚ¼Óæģ·“Ó¦ĖŁĀŹ£¬µ«½µµĶĮĖ·“Ó¦ĪļµÄ×Ŗ»ÆĀŹ£¬ŹŹŅĖĢõ¼žµÄŃ”ŌńŠčŅŖ×ŪŗĻæ¼ĀĒ”£
“š°ø£ŗ(1)450”ę””(2)1”Į105Pa””ŅņĪŖ³£Ń¹ĻĀSO2µÄ×Ŗ»ÆĀŹŅŃ¾ŗÜøߣ¬Čō²ÉÓĆ½Ļ“óµÄŃ¹Ē棬SO2µÄ×Ŗ»ÆĀŹĢįøßŗÜÉŁ£¬µ«ŠčŅŖ¶ÆĮ¦øü“󣬶ŌÉč±øµÄŅŖĒóøüøß””(3)ŅņĪŖSO2µÄ×Ŗ»ÆĀŹ±Č½Ļøߣ¬“ļµ½Ę½ŗāŗóµÄ»ģŗĻĘųĢåÖŠSO2µÄÓąĮæŗÜÉŁ£¬¹Ź²»ŠčŅŖ·ÖĄėSO3”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ij¶žŌŖĖį(»ÆѧŹ½ÓĆH2A±ķŹ¾)ŌŚĖ®ČÜŅŗÖŠµÄµēĄė·½³ĢŹ½ŹĒH2A===H£«£«HA££»HA£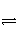

H£«£«A2£”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)Na2AČÜŅŗĻŌ________(Ģī”°ĖįŠŌ”±”¢”°ÖŠŠŌ”±»ņ”°¼īŠŌ”±)£¬ĄķÓÉŹĒ(ÓĆĄė×Ó·½³ĢŹ½±ķŹ¾)________________________________________________________________________”£
(2)Čō0.1 mol·L£1 NaHAČÜŅŗµÄpH£½2£¬Ōņ0.1 mol·L£1 H2AČÜŅŗÖŠĒāĄė×ÓµÄĪļÖŹµÄĮæÅضČæÉÄÜ__________0.11 mol·L£1(Ģī”°£¾”±”¢”°£½”±»ņ”°£¼”±)£¬ĄķÓÉŹĒ
________________________________________________________________________
__________________ӣ
(3)0.1 mol·L£1 NaHAµÄČÜŅŗÖŠø÷ÖÖĄė×ÓÅضČÓɓ󵽊”µÄĖ³ŠņŹĒ
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ņ»ÖÖ³ĪĒåĶøĆ÷µÄČÜŅŗÖŠ£¬æÉÄÜŗ¬ÓŠĻĀĮŠĄė×Ó£ŗK£«”¢Fe3£«”¢Ba2£«”¢Al3£«”¢NH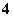 ”¢Cl£”¢NO
”¢Cl£”¢NO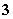 ”¢HCO
ӢHCO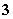 ӢSO
”¢SO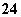 ”£ĻÖ×öŅŌĻĀŹµŃé£ŗ
”£ĻÖ×öŅŌĻĀŹµŃé£ŗ
(1)½«ČÜŅŗµĪŌŚĄ¶É«ŹÆČļŹŌÖ½ÉĻ£¬ŹŌÖ½³ŹŗģÉ«£»
(2)ȔɣĮæČÜŅŗ£¬¼ÓČėÓĆĻ”HNO3Ėį»ÆµÄBaCl2ČÜŅŗ£¬²śÉś°×É«³Įµķ£»
(3)½«(2)ÖŠµÄ³Įµķ¹żĀĖ£¬ĻņĀĖŅŗÖŠ¼ÓČėAgNO3ČÜŅŗ£¬²śÉś°×É«³Įµķ£»
(4)ĮķČ”ČÜŅŗ£¬ÖšµĪ¼ÓČėNaOHČÜŅŗÖĮ¹żĮ棬ֻ擵½ÓŠŗģŗÖÉ«³ĮµķÉś³É£¬ĒŅ³ĮµķÖŹĮæ²»¼õÉŁ”£
ÓÉ“ĖæÉŅŌĶʶĻ£ŗ
ČÜŅŗÖŠæĻ¶Ø“ęŌŚµÄĄė×ÓÓŠ_________________________________________________£»
ČÜŅŗÖŠæĻ¶Ø²»“ęŌŚµÄĄė×ÓÓŠ________________________________________________£»
ČÜŅŗÖŠ²»ÄÜČ·¶ØŹĒ·ń“ęŌŚµÄĄė×ÓÓŠ_________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠŹµŃé²Ł×÷ÖŠ£ŗ¢ŁČ”ŅŗĢåŹŌ¼Į””¢ŚČ”¹ĢĢåŹŌ¼Į””¢ŪČܽā
¢Ü¹żĀĖ””¢ŻÕō·¢£¬Ņ»¶ØŅŖÓƵ½²£Į§°ōµÄŹĒ(””””)
A£®¢Ł¢Ś¢Ū”””””””””””””” B£®¢Ś¢Ū¢Ü
C£®¢Ł¢Ś¢Ż D£®¢Ū¢Ü¢Ż
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚŗćĪĀŗćČŻĢõ¼žĻĀ£¬ÄÜŹ¹A(g)£«B(g)C(g)£«D(g)Õż·“Ó¦ĖŁĀŹŌö“óµÄ“ėŹ©ŹĒ(””””)
¢Ł¼õŠ”C»ņDµÄÅØ¶Č ¢ŚŌö“óDµÄÅضČ
¢Ū¼õŠ”BµÄÅØ¶Č ¢ÜŌö“óA»ņBµÄÅضČ
A£®¢Ł¢Ś B£®¢Ś¢Ū
C£®¢Ł¢Ü D£®¢Ś¢Ü
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½«H2(g)ŗĶBr2(g)³äČėŗćČŻĆܱÕČŻĘ÷£¬ŗćĪĀĻĀ·¢Éś·“Ó¦H2(g)£«Br2(g)2HBr(g)””¦¤H<0£¬Ę½ŗāŹ±Br2(g)µÄ×Ŗ»ÆĀŹĪŖa£»Čō³õŹ¼Ģõ¼žĻąĶ¬£¬¾ųČČĻĀ½ųŠŠÉĻŹö·“Ó¦£¬Ę½ŗāŹ±Br2(g)µÄ×Ŗ»ÆĀŹĪŖb”£aÓėbµÄ¹ŲĻµŹĒ(””””)
A£®a>b B£®a£½b
C£®a<b D£®ĪŽ·ØČ·¶Ø
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ
A£® 126C ”¢136C ”¢146C ĪŖĢ¼ŌŖĖŲµÄČżÖÖŗĖĖŲ”£ŌŖĖŲÖÜĘŚ±ķÖŠĢ¼µÄĻą¶ŌŌ×ÓÖŹĮæĪŖ12.01£¬ĖµĆ÷×ŌČ»½ēÖŠµÄĢ¼Ö÷ŅŖŅŌ126C µÄŗĖĖŲŠĪŹ½“ęŌŚ”£146CĪŖ·ÅÉäŠŌŗĖĖŲ£¬æÉÓĆÓŚĶ¬Ī»ĖŲŹ¾×Ł
B£®Ęū³µĪ²Ęų“ß»Æ×Ŗ»Æ×°ÖĆæɽ«Ī²ĘųÖŠµÄNOŗĶCOµČÓŠŗ¦ĘųĢå×Ŗ»ÆĪŖN2ŗĶCO2£¬øĆ×°ÖĆÖŠµÄ“߻ƼĮæɽµµĶNOŗĶCO·“Ó¦µÄ»ī»ÆÄÜ£¬ÓŠĄūÓŚĢįøßøĆ·“Ó¦µÄĘ½ŗā×Ŗ»ÆĀŹ
C£®µĄ¶ū¶Ł”¢ĢĄÄ·Éś”¢Ā¬ÉŖø£”¢²£¶ūµČæĘѧ¼ŅµÄŃŠ¾æ²»¶ĻøüŠĀČĖĆĒ¶ŌŌ×Ó½į¹¹µÄČĻŹ¶
D£®µŲ¹µÓĶÓÉÓŚ»ģÓŠŅ»Š©¶ŌČĖĢåÓŠŗ¦µÄŌÓÖŹ¶ų²»ÄÜŹ³ÓĆ£¬æɼӹ¤ÖĘ³ÉÉśĪļ²ńÓĶ£¬ÉśĪļ²ńÓĶµÄ³É·ÖÓė“ÓŹÆÓĶÖŠĢįČ”µÄ²ńÓĶ³É·Ö²»Ķ¬
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 £Ø1£©¾Ż±ØµĄŅŌÅšĒā»ÆŗĻĪļNaBH4£ØHµÄ»ÆŗĻ¼ŪĪŖ-1¼Ū£©ŗĶH2O2×÷ŌĮĻµÄČ¼ĮĻµē³Ų£¬æÉÓĆ×÷ĶØŠÅĪĄŠĒµē”£øŗ¼«²ÄĮĻ²ÉÓĆPt/C£¬Õż¼«²ÄĮĻ²ÉÓĆMnO2£¬Ę乤×÷ŌĄķČēĶ¼ĖłŹ¾”£Š“³öøƵē³Ų·ÅµēŹ±øŗ¼«µÄµē¼«·“Ó¦Ź½£ŗ
£Ø1£©¾Ż±ØµĄŅŌÅšĒā»ÆŗĻĪļNaBH4£ØHµÄ»ÆŗĻ¼ŪĪŖ-1¼Ū£©ŗĶH2O2×÷ŌĮĻµÄČ¼ĮĻµē³Ų£¬æÉÓĆ×÷ĶØŠÅĪĄŠĒµē”£øŗ¼«²ÄĮĻ²ÉÓĆPt/C£¬Õż¼«²ÄĮĻ²ÉÓĆMnO2£¬Ę乤×÷ŌĄķČēĶ¼ĖłŹ¾”£Š“³öøƵē³Ų·ÅµēŹ±øŗ¼«µÄµē¼«·“Ó¦Ź½£ŗ
”ų ”£
£Ø2£©»š¼ż·¢Éä³£ŅŌŅŗĢ¬ėĀ£ØN2H4£©ĪŖČ¼ĮĻ£¬ŅŗĢ¬¹żŃõ»ÆĒā
ĪŖÖśČ¼¼Į”£
ŅŃÖŖ£ŗ N2H4£Øl£© + O2£Øg£© £½ N2£Øg£©+ 2H2O£Øl£© ”÷H = ØC 534 kJ”¤mol”Ŗ1
H2O2£Øl£©= H2O£Øl£© + 1/2O2£Øg£© ”÷H = ØC 98.6 kJ”¤mol”Ŗ1
Š“³ö³£ĪĀĻĀ£¬N2H4£Øl£© Óė H2O2£Øl£©·“Ӧɜ³ÉN2ŗĶH2OµÄČČ»Æѧ·½³ĢŹ½ ”ų ”£
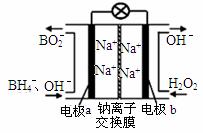
 £Ø3£©O3æÉÓɳōŃõ·¢ÉśĘ÷£ØŌĄķČēÓŅĶ¼ĖłŹ¾£©µē½āĻ”ĮņĖįÖʵƔ£
£Ø3£©O3æÉÓɳōŃõ·¢ÉśĘ÷£ØŌĄķČēÓŅĶ¼ĖłŹ¾£©µē½āĻ”ĮņĖįÖʵƔ£
¢ŁĶ¼ÖŠŅõ¼«ĪŖ ”ų £ØĢī”°A”±»ņ”°B”±£©”£
¢ŚČōC“¦ĶØČėO 2£¬ŌņA¼«µÄµē¼«·“Ó¦Ź½ĪŖ£ŗ ”ų
£Ø4£©ĻņŅ»ĆܱÕČŻĘ÷ÖŠ³äČėŅ»¶ØĮæŅ»Ńõ»ÆĢ¼øśĖ®ÕōĘų·¢Éś·“Ó¦ CO(g)+H2O(g) CO2(g)+H2(g)£¬ĻĀĮŠĒéæöĻĀÄÜÅŠ¶ĻøĆ·“Ó¦Ņ»¶Ø“ļµ½Ę½ŗāדĢ¬µÄŹĒ____ ”ų _____£ØŃ”Ģī±ąŗÅ£©”£
CO2(g)+H2(g)£¬ĻĀĮŠĒéæöĻĀÄÜÅŠ¶ĻøĆ·“Ó¦Ņ»¶Ø“ļµ½Ę½ŗāדĢ¬µÄŹĒ____ ”ų _____£ØŃ”Ģī±ąŗÅ£©”£
A£®vÕż£ØH2O) = vÄę£ØH2)
B£®ČŻĘ÷ÖŠĘųĢåµÄŃ¹Ēæ²»ŌŁ·¢Éśøıä
C£®H2OµÄĢå»ż·ÖŹż²»ŌŁøıä
D£®ČŻĘ÷ÖŠCO2ŗĶH2µÄĪļÖŹµÄĮæÖ®±Č²»ŌŁ·¢Éśøıä
E£®ČŻĘ÷ÖŠĘųĢåµÄĆÜ¶Č²»ŌŁ·¢Éśøıä
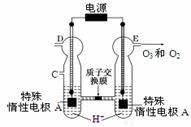
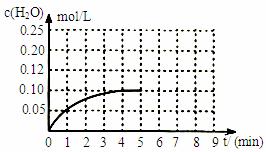 £Ø5£©ĪĀ¶ČT1Ź±£¬ŌŚŅ»Ģå»żĪŖ2LµÄĆܱÕČŻ»żÖŠ,¼ÓČė0.4molCO2ŗĶ0.4molµÄH2,·“Ó¦ÖŠc(H2O)µÄ±ä»ÆĒéæöČēĶ¼ĖłŹ¾,T1Ź±·“Ó¦CO(g)+H2O(g)
£Ø5£©ĪĀ¶ČT1Ź±£¬ŌŚŅ»Ģå»żĪŖ2LµÄĆܱÕČŻ»żÖŠ,¼ÓČė0.4molCO2ŗĶ0.4molµÄH2,·“Ó¦ÖŠc(H2O)µÄ±ä»ÆĒéæöČēĶ¼ĖłŹ¾,T1Ź±·“Ó¦CO(g)+H2O(g) CO2(g)+H2(g)µŚ4·ÖÖÓ“ļµ½Ę½ŗā”£ŌŚµŚ5·ÖÖÓŹ±ĻņĢåĻµÖŠĶ¬Ź±ŌŁ³äČė0.1molCOŗĶ0.1molH2£ØĘäĖūĢõ¼ž²»±ä£©£¬ĒėŌŚÓŅĶ¼ÖŠ»³öµŚ5·ÖÖÓµ½9·ÖÖÓc(H2O)ÅØ¶Č±ä»ÆĒ÷ŹĘµÄĒśĻß”£
CO2(g)+H2(g)µŚ4·ÖÖÓ“ļµ½Ę½ŗā”£ŌŚµŚ5·ÖÖÓŹ±ĻņĢåĻµÖŠĶ¬Ź±ŌŁ³äČė0.1molCOŗĶ0.1molH2£ØĘäĖūĢõ¼ž²»±ä£©£¬ĒėŌŚÓŅĶ¼ÖŠ»³öµŚ5·ÖÖÓµ½9·ÖÖÓc(H2O)ÅØ¶Č±ä»ÆĒ÷ŹĘµÄĒśĻß”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½ńÓŠĻĀĮŠĘųĢå£ŗH2”¢Cl2”¢HCl”¢NH3”¢NO”¢H2S”¢SO2£¬ÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆ½ųŠŠŹµŃ飬ĢīæÕĻĀĮŠæÕ°×£ŗ
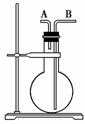
(1)ÉÕĘæøÉŌļŹ±£¬“ÓAæŚ½ųĘųæÉŹÕ¼ÆµÄĘųĢåŹĒ________£¬“ÓBæŚ½ųĘųæÉŹÕ¼ÆµÄĘųĢåŹĒ______________”£
(2)ÉÕĘæÖŠ³äĀśĖ®Ź±£¬æÉÓĆĄ“²āĮæ________µČĘųĢåµÄĢå»ż”£
(3)µ±ÉÕĘæ֊װČėĻ“Ņŗ£¬ÓĆÓŚĻ“ĘųŹ±£¬ĘųĢåÓ¦“Ó________æŚ½ųČėÉÕĘ攣
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com