
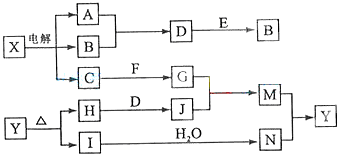
£Ø12·Ö£©ĻĀĮŠæņĶ¼ÖŠµÄA”ŖJŹĒ֊ѧ»Æѧ֊³£¼ūµÄ°ĖÖÖĪļÖŹ£¬ĖłÓŠĪļÖŹ¾łÓɶĢÖÜĘŚŌŖĖŲ×é³É£¬ŅŃÖŖ³£ĪĀ”¢³£Ń¹ĻĀDĪŖĪŽÉ«ŅŗĢ壬 C”¢E”¢G¾łĪŖĘųĢåµ„ÖŹ£¬BĪŖ½šŹō£¬IŹĒÓÉ3øöŌ×Ó×é³ÉµÄŅ»ŌŖŗ¬ŃõČõĖį·Ö×Ó£¬ A”ŖJæÉ·¢ÉśČēĻĀ×Ŗ»Æ£ŗ
ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
¢ÅGµÄĆū³ĘĪŖ £¬FµÄµē×ÓŹ½ĪŖ ”£
¢Ę15.6gAÓėDĶźČ«·“Ó¦£¬×ŖŅʵĵē×ÓµÄĪļÖŹµÄĮæĪŖ ”£
¢Ē³£ĪĀĻĀ£¬AĪŖŅ»ÖÖ É«µÄ¹ĢĢ壬ĖüµÄŅ»ÖÖÖŲŅŖÓĆĶ¾ŹĒ  ”£
ӣ
¢ČŠ“³öD”¢G·“Ó¦×Ŗ»ÆĪŖIŗĶJµÄĄė×Ó·½ ³ĢŹ½
³ĢŹ½  ”£
ӣ
 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğ¹ćĪ÷Ź”øßČżÉĻѧʌµŚČż“ĪŌĀæ¼»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø12·Ö£©ĻĀĮŠæņĶ¼ÖŠµÄA”ŖJŹĒ֊ѧ»Æѧ֊³£¼ūµÄ°ĖÖÖĪļÖŹ£¬ĖłÓŠĪļÖŹ¾łÓɶĢÖÜĘŚŌŖĖŲ×é³É£¬ŅŃÖŖ³£ĪĀ”¢³£Ń¹ĻĀDĪŖĪŽÉ«ŅŗĢ壬 C”¢E”¢G¾łĪŖĘųĢåµ„ÖŹ£¬BĪŖ½šŹō£¬IŹĒÓÉ3øöŌ×Ó×é³ÉµÄŅ»ŌŖŗ¬ŃõČõĖį·Ö×Ó£¬ A”ŖJæÉ·¢ÉśČēĻĀ×Ŗ»Æ£ŗ
ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
¢ÅGµÄĆū³ĘĪŖ £¬FµÄµē×ÓŹ½ĪŖ ”£
¢Ę15.6gAÓėDĶźČ«·“Ó¦£¬×ŖŅʵĵē×ÓµÄĪļÖŹµÄĮæĪŖ ”£
¢Ē³£ĪĀĻĀ£¬AĪŖŅ»ÖÖ É«µÄ¹ĢĢ壬ĖüµÄŅ»ÖÖÖŲŅŖÓĆĶ¾ŹĒ ”£
¢ČŠ“³öD”¢G·“Ó¦×Ŗ»ÆĪŖIŗĶJµÄĄė×Ó·½³ĢŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø12·Ö£©ĻĀĮŠæņĶ¼ÖŠµÄA”ŖJŹĒ֊ѧ»Æѧ֊³£¼ūµÄ°ĖÖÖĪļÖŹ£¬ĖłÓŠĪļÖŹ¾łÓɶĢÖÜĘŚŌŖĖŲ×é³É£¬ŅŃÖŖ³£ĪĀ”¢³£Ń¹ĻĀDĪŖĪŽÉ«ŅŗĢ壬 C”¢E”¢G¾łĪŖĘųĢåµ„ÖŹ£¬BĪŖ½šŹō£¬IŹĒÓÉ3øöŌ×Ó×é³ÉµÄŅ»ŌŖŗ¬ŃõČõĖį·Ö×Ó£¬ A”ŖJæÉ·¢ÉśČēĻĀ×Ŗ»Æ£ŗ
ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
¢ÅGµÄĆū³ĘĪŖ £¬FµÄµē×ÓŹ½ĪŖ ”£
¢Ę15.6gAÓėDĶźČ«·“Ó¦£¬×ŖŅʵĵē×ÓµÄĪļÖŹµÄĮæĪŖ ”£
¢Ē³£ĪĀĻĀ£¬AĪŖŅ»ÖÖ É«µÄ¹ĢĢ壬ĖüµÄŅ»ÖÖÖŲŅŖÓĆĶ¾ŹĒ ”£
¢ČŠ“³öD”¢G·“Ó¦×Ŗ»ÆĪŖIŗĶJµÄĄė×Ó·½³ĢŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com