
��1���Խ���AlF3�� AlCl3���۷е���ںܴ�����ԭ��
��2����500 K��1.01��105 Paʱ,���AlCl3�������ܶ�Ϊ
��3�������һ��ʵ�鷽�����ж�AlCl3�����ӻ����ﻹ�ǹ��ۻ����
������������Ŀ�и�������ʾ�ش�
�𰸣���1��AlF3�������Ӽ����ɵ����Ӿ��壬��AlCl3�Ƿ��Ӿ��壬���Ӽ���ڵ��Ƿ��»�������ǰ�����۷е���ں��ߡ�
��2���Ȼ�������Է�������Ϊ11.92��22.4=267���ɴ˿��Լ��������ʽΪAl2Cl6��Alԭ����sp3�ӻ���Alԭ����������������ӣ�ռ���ĸ�sp3�ӻ�����е�������������Clԭ���γɹ��ۼ�������һ��sp3�ӻ����û�е��ӣ������һ��Clԭ���ϵŶԵ����γ���λ��������ͼ����
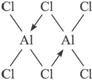
��3�������ڵ��Ȼ����Ƿ磬�����磬�����ӻ�����������磬�ǹ��ۻ����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����



| ||
| ||

| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪���о�����۵㣺NaCl��801�� AlF3��1291�� AlCl3��190�� BCl3��107��
����Al2O3��2045�� CO2����56.6�� SiO2��1723�� �ݴ��ж�����˵���������
A����Ԫ�غ�����ɵľ������е���ԭ�Ӿ���
B���Ѹ�����������ֻ��BCl3��CO2�Ƿ��Ӿ���
C��ͬ��Ԫ�ص�����������γɲ�ͬ���͵ľ���
D����ͬ��Ԫ�ص�����������γ���ͬ���͵ľ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ӱ�ʡ�߶���ѧ���������Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪���о�����۵㣺NaCl��801�� AlF3��1291�� AlCl3��190�� BCl3��107��
����Al2O3��2045�� CO2����56.6�� SiO2��1723�� �ݴ��ж�����˵���������
A����Ԫ�غ�����ɵľ������е���ԭ�Ӿ���
B���Ѹ�����������ֻ��BCl3��CO2�Ƿ��Ӿ���
C��ͬ��Ԫ�ص�����������γɲ�ͬ���͵ľ���
D����ͬ��Ԫ�ص�����������γ���ͬ���͵ľ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com