
��13�֣�ʵʩ�Լ�����Դ�˷Ѻͽ��ͷ����ŷ�Ϊ�������ݵĽ��ܼ������ߣ���Ӧ��ȫ���������⡢������Դ��Լ�͡������Ѻ������ı�Ȼѡ������ҵ�ķ�չ������Ϲ��ҽ��ܼ��ŵ�����Ҫ����ͼ��ú������ҵ����һ���֣���������ѧ֪ʶ������������⣺
��1���ò�ҵ�������ڸ��нγ������ڵ��������Է����еķ�Ӧ�ǣ�
��2����֪�ò�ҵ����ij��Ӧ��ƽ�����ʽΪ��
������Ӧ�Ļ�ѧ��ӦΪ��
��3����֪��һ���¶��£�
C��s��+CO2��g�� 2CO��g��ƽ�ⳣ��K1��
CO��g��+H2O��g H2��g��+CO2��g��ƽ�ⳣ��K2��
C��s��+H2O��g�� CO��g��+H2��g�� ƽ�ⳣ��K3��
��K1��K2��K3֮��Ĺ�ϵ�ǣ� ��
��4��ú����ͨ��ͨ���о���ͬ�¶���ƽ�ⳣ���Խ������ʵ�����⡣��֪�������һ����̼��ˮ�������뷴Ӧ��ʱ���ᷢ�����·�Ӧ��CO(g)+H2O(g) H2(g)+CO2(g)���÷�Ӧƽ�ⳣ�����¶ȵı仯���±���ʾ��
| �¶�/�� | 400 | 500 | 800 |
| ƽ�ⳣ��K | 9.94 | 9 | 1 |
�÷�Ӧ������Ӧ������ ��Ӧ������ȡ����ȡ���������500��ʱ���У�����ʼʱCO��H2O����ʼŨ�Ⱦ�Ϊ0.020mol/L���ڸ������£�CO��ƽ��ת����Ϊ�� ��
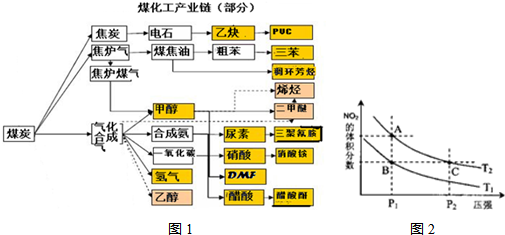
��5������ͼ���������������������ᣬ�˹������漰���������NO��NO2��N2O4�ȡ��Է�ӦN2O4(g) 2NO2(g) ��H��0���¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ������������������
A��A��C����ķ�Ӧ���ʣ�A��C
B��A��C�����������ɫ��A�Cdz
C��B��C����������ƽ����Է���������B��C
D����״̬B��״̬A�������ü��ȵķ���
E��A��C����Ļ�ѧƽ�ⳣ����A��C
��6��0.2mol/L��NaOH��0.4mol/L��������ҵ����һ��Ʒ�����������Һ�������Ϻ���Һ�и����ӵ����ʵ���Ũ�ȴӴ�С��˳���� ��
 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| c(H2)c(CO) |
| c(H2O) |
| �¶�/�� | 400 | 500 | 800 |
| ƽ�ⳣ��K | 9.94 | 9 | 1 |
 ��
�� ��ʵ����N-N������Ϊ167kJ?mol-1��NO2�е������ļ���Ϊ466kJ?mol-1��N2O4�е������ļ���Ϊ438.5kJ?mol-1����д��NO2ת��ΪN2O4���Ȼ�ѧ����ʽ
��ʵ����N-N������Ϊ167kJ?mol-1��NO2�е������ļ���Ϊ466kJ?mol-1��N2O4�е������ļ���Ϊ438.5kJ?mol-1����д��NO2ת��ΪN2O4���Ȼ�ѧ����ʽ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
ʵʩ�Լ�����Դ�˷Ѻͽ��ͷ����ŷ�Ϊ�������ݵĽ��ܼ������ߣ���Ӧ��ȫ���������⡢������Դ��Լ�͡������Ѻ������ı�Ȼѡ������ҵ�ķ�չ������Ϲ��ҽ��ܼ��ŵ�����Ҫ����������ѧ֪ʶ������������⣺
��1����֪ij��Ӧ��ƽ�����ʽΪ��

������Ӧ�Ļ�ѧ��ӦΪ��
��2����֪��һ���¶��£�
C��s��+CO2��g�� 2CO��g��ƽ�ⳣ��K1��
CO��g��+H2O��g H2��g��+CO2��g��ƽ�ⳣ��K2��
C��s��+H2O��g�� CO��g��+H2��g�� ƽ�ⳣ��K3��
��K1��K2��K3֮��Ĺ�ϵ�ǣ� ��
��3��ú����ͨ��ͨ���о���ͬ�¶���ƽ�ⳣ���Խ������ʵ�����⡣��֪�������һ����̼��ˮ�������뷴Ӧ��ʱ���ᷢ�����·�Ӧ��CO(g)+H2O(g) H2(g)+CO2(g)���÷�Ӧƽ�ⳣ�����¶ȵı仯���±���ʾ��
| �¶�/�� | 400 | 500 | 800 |
| ƽ�ⳣ��K | 9.94 | 9 | 1 |
�÷�Ӧ������Ӧ������ ��Ӧ������ȡ����ȡ���������500��ʱ���У�����ʼʱCO��H2O����ʼŨ�Ⱦ�Ϊ0.020mol/L���ڸ������£�CO��ƽ��ת����Ϊ�� ��
��4���Ӱ����������������ᣬ�˹������漰���������NO��NO2��N2O4�ȡ��Է�ӦN2O4(g)2NO2(g) ��H��0���¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ������������������
A��A��C����ķ�Ӧ���ʣ�A��C
B��A��C�����������ɫ��A�Cdz
C��B��C����������ƽ����Է���������B��C
D����״̬B��״̬A�������ü��ȵķ���
E��A��C����Ļ�ѧƽ�ⳣ����A��C
��5��0.2mol/L��NaOH��0.4mol/L���������Һ�������Ϻ���Һ�и����ӵ����ʵ���Ũ�ȴӴ�С��˳���� ��
(6)��ҵ����Na2SO3����β���е�SO2��������ͼװ�õ��(���Ե缫)NaHSO3��ȡH2SO4�������缫��Ӧʽ ���������ݳ�����ijɷ�Ϊ __________(�ѧʽ)��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
ʵʩ�Լ�����Դ�˷Ѻͽ��ͷ����ŷ�Ϊ�������ݵĽ��ܼ������ߣ���Ӧ��ȫ���������⡢������Դ��Լ�͡������Ѻ������ı�Ȼѡ������ҵ�ķ�չ������Ϲ��ҽ��ܼ��ŵ�����Ҫ����������ѧ֪ʶ������������⣺
��1����֪ij��Ӧ��ƽ�����ʽΪ��

������Ӧ�Ļ�ѧ��ӦΪ��
��2����֪��һ���¶��£�
C��s��+CO2��g�� 2CO��g��ƽ�ⳣ��K1��
CO��g��+H2O��g H2��g��+CO2��g��ƽ�ⳣ��K2��
C��s��+H2O��g�� CO��g��+H2��g�� ƽ�ⳣ��K3��
��K1��K2��K3֮��Ĺ�ϵ�ǣ� ��
��3��ú����ͨ��ͨ���о���ͬ�¶���ƽ�ⳣ���Խ������ʵ�����⡣��֪�������һ����̼��ˮ�������뷴Ӧ��ʱ���ᷢ�����·�Ӧ��CO(g)+H2O(g) H2(g)+CO2(g)���÷�Ӧƽ�ⳣ�����¶ȵı仯���±���ʾ��
| �¶�/�� | 400 | 500 | 800 |
| ƽ�ⳣ��K | 9.94 | 9 | 1 |
�÷�Ӧ������Ӧ������ ��Ӧ������ȡ����ȡ���������500��ʱ���У�����ʼʱCO��H2O����ʼŨ�Ⱦ�Ϊ0.020mol/L���ڸ������£�CO��ƽ��ת����Ϊ�� ��
��4���Ӱ����������������ᣬ�˹������漰���������NO��NO2��N2O4�ȡ��Է�ӦN2O4(g)2NO2(g) ��H��0���¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ������������������
A��A��C����ķ�Ӧ���ʣ�A��C
B��A��C�����������ɫ��A�Cdz
C��B��C����������ƽ����Է���������B��C
D����״̬B��״̬A�������ü��ȵķ���
E��A��C����Ļ�ѧƽ�ⳣ����A��C
��5��0.2mol/L��NaOH��0.4mol/L���������Һ�������Ϻ���Һ�и����ӵ����ʵ���Ũ�ȴӴ�С��˳���� ��
(6)��ҵ����Na2SO3����β���е�SO2��������ͼװ�õ��(���Ե缫)NaHSO3��ȡH2SO4�������缫��Ӧʽ ���������ݳ�����ijɷ�Ϊ __________(�ѧʽ)��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭���ϸ߶��С����������ѧ�����꼶ȫ��ģ�⣨���ۣ���ѧ���� ���ͣ������
ʵʩ�Լ�����Դ�˷Ѻͽ��ͷ����ŷ�Ϊ�������ݵĽ��ܼ������ߣ���Ӧ��ȫ���������⡢������Դ��Լ�͡������Ѻ������ı�Ȼѡ������ҵ�ķ�չ������Ϲ��ҽ��ܼ��ŵ�����Ҫ����������ѧ֪ʶ������������⣺
��1����֪ij��Ӧ��ƽ�����ʽΪ��

������Ӧ�Ļ�ѧ��ӦΪ��
��2����֪��һ���¶��£�
C��s��+CO2��g��  2CO��g��ƽ�ⳣ��K1��
2CO��g��ƽ�ⳣ��K1��
CO��g��+H2O��g  H2��g��+CO2��g��ƽ�ⳣ��K2��
H2��g��+CO2��g��ƽ�ⳣ��K2��
C��s��+H2O��g�� CO��g��+H2��g�� ƽ�ⳣ��K3��
CO��g��+H2��g�� ƽ�ⳣ��K3��
��K1��K2��K3֮��Ĺ�ϵ�ǣ� ��
��3��ú����ͨ��ͨ���о���ͬ�¶���ƽ�ⳣ���Խ������ʵ�����⡣��֪�������һ����̼��ˮ�������뷴Ӧ��ʱ���ᷢ�����·�Ӧ��CO(g)+H2O(g)  H2(g)+CO2(g)���÷�Ӧƽ�ⳣ�����¶ȵı仯���±���ʾ��
H2(g)+CO2(g)���÷�Ӧƽ�ⳣ�����¶ȵı仯���±���ʾ��
|
�¶�/�� |
400 |
500 |
800 |
|
ƽ�ⳣ��K |
9.94 |
9 |
1 |
�÷�Ӧ������Ӧ������ ��Ӧ������ȡ����ȡ���������500��ʱ���У�����ʼʱCO��H2O����ʼŨ�Ⱦ�Ϊ0.020mol/L���ڸ������£�CO��ƽ��ת����Ϊ�� ��
��4���Ӱ����������������ᣬ�˹������漰���������NO��NO2��N2O4�ȡ��Է�ӦN2O4(g)
 2NO2(g) ��H��0���¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ������������������
2NO2(g) ��H��0���¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ������������������

A��A��C����ķ�Ӧ���ʣ�A��C
B��A��C�����������ɫ��A�Cdz
C��B��C����������ƽ����Է���������B��C
D����״̬B��״̬A�������ü��ȵķ���
E��A��C����Ļ�ѧƽ�ⳣ����A��C
��5��0.2mol/L��NaOH��0.4mol/L���������Һ�������Ϻ���Һ�и����ӵ����ʵ���Ũ�ȴӴ�С��˳���� ��
(6)��ҵ����Na2SO3����β���е�SO2��������ͼװ�õ��(���Ե缫)NaHSO3��ȡH2SO4�������缫��Ӧʽ ���������ݳ�����ijɷ�Ϊ __________(�ѧʽ)��

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com