
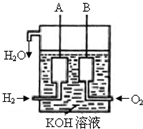
 ��֪��25��C��1.013��105Pa�£�1mol������ȫȼ������Һ̬ˮ�ų�285kJ����������ش��������⣺
��֪��25��C��1.013��105Pa�£�1mol������ȫȼ������Һ̬ˮ�ų�285kJ����������ش��������⣺
| ||


 A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д� ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| c(H+)c(HCO-3) |
| c(H2CO3) |
| c(H+)c(HCO-3) |
| c(H2CO3) |
| ���ܵ���� | AgI | AgOH | Ag2S | PbI2 | Pb��OH��2 | PbS |
| KSP | 8.3��10-17 | 5.6��10-8 | 6.3��10-50 | 7.1��10-9 | 1.2��10-15 | 3.4��10-28 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 I����������20mL0.1mol?L-1Na2CO3��Һ����μ���0.1mol?L-1 HCl��Һ40mL����Һ��pH���ͣ���ʱ��Һ�к�̼Ԫ�ص������ʵ���Ũ�ȵİٷֺ��������ᣩҲ�����仯��CO2���ݳ�δ����������ͼ��ʾ���ش��������⣺
I����������20mL0.1mol?L-1Na2CO3��Һ����μ���0.1mol?L-1 HCl��Һ40mL����Һ��pH���ͣ���ʱ��Һ�к�̼Ԫ�ص������ʵ���Ũ�ȵİٷֺ��������ᣩҲ�����仯��CO2���ݳ�δ����������ͼ��ʾ���ش��������⣺c(
| ||
c(
|
 H++HA-����HA-
H++HA-����HA- H++A2-
H++A2-�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
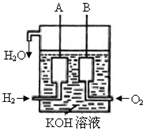
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com