
������ڻ�ѧѧ�Ƶķ�չ�����˷dz���Ҫ�����á����з������������
�ٸ���̼��������ˮ�ʼ��ԣ���֪̼���Ƽ������Σ������ڼ�
�ڸ��ݷ�Ӧ���Ƿ��е��ӵ�ת�ƣ�����ѧ��Ӧ��Ϊ������ԭ��Ӧ�ͷ�������ԭ��Ӧ
�۸��ݷ�ɢϵ�Ƿ���ж����������ɢϵ��Ϊ��Һ�����������Һ
�ܸ�����ˮ��Һ�л�����״̬���ܷ磬���������Ϊ����ʺͷǵ����
A���٢ۡ����������������� ��B���ڢ�
C���٢� D���ۢ�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ܹ������������
A����ɫ��Һ�У�Fe3+��Na+��NO3����Cl��
B��pH=0����Һ�У�Fe2+��NO3����Cl����HCO3��
C����MnO4������Һ�У�Fe3+��SO42����NO3����Mg2+
D����������������������Һ��Mg2+��NO3����K+��Cl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧ����С���о��ϳɰ���N2(g)+3H2(g)  2NH3(g)����H<0,��������������ʱ���ı�ijһ����ʱ�Ի�ѧƽ���Ӱ�죬�õ�����ͼ�����¶�Ӧѡ������ȷ���ǣ� ��
2NH3(g)����H<0,��������������ʱ���ı�ijһ����ʱ�Ի�ѧƽ���Ӱ�죬�õ�����ͼ�����¶�Ӧѡ������ȷ���ǣ� ��
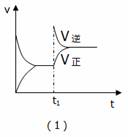


A.��1����Ӧ���ǣ���t1ʱ��ѹ�����£�ͨ��NH3
B. ��2����Ӧ���ǣ������ں��ݲ�ͬ�¶��µİٷֺ���
��2����Ӧ���ǣ������ں��ݲ�ͬ�¶��µİٷֺ���
C.��3����Ӧ���ǣ��ں��������£���Ӧ�������¶ȵĹ�ϵ
D.��4����Ӧ���ǣ�N2ת�������¶ȣ�T1>T2����ѹǿ�Ĺ�ϵ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�¶��£����ڷ�ӦN2(g)��3H2(g)  2NH3(g)����H����92.4 kJ��mol��1��N2��ƽ��ת����(��)����ϵ��ѹǿ(p)�Ĺ�ϵ��ͼ��ʾ������˵����ȷ����
2NH3(g)����H����92.4 kJ��mol��1��N2��ƽ��ת����(��)����ϵ��ѹǿ(p)�Ĺ�ϵ��ͼ��ʾ������˵����ȷ����
A����1 mol������3 mol����������1 L�ܱ������з�����Ӧ���ų������� Ϊ92.4 kJ
Ϊ92.4 kJ
B��ƽ��״̬��A�䵽Bʱ��ƽ�ⳣ��K(A)��K(B)
C��������Ӧ�ڴﵽƽ����� ��ѹǿ��H2��ת��������
��ѹǿ��H2��ת��������
D�������¶ȣ�ƽ�����淴Ӧ�����ƶ���˵���淴Ӧ������������Ӧ���ʼ�С

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ��������ᷢչ��������Ҫ�����ã�չ��δ������ѧ��ѧ����ʮ�ֹ�����̽���ռ䡣�ִ���ѧ���漰���о�������
A�������µ���Դ B���ϳ��µ�����
C���ռ���ʽ��������ϵ D�����λ�����Ⱦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼ��ʾ���Թ���ʢ��ˮ������a��ʢ�и����Na2O2��ĩ��U�ι���ע��dz��ɫ��ˮ������������Ƥ����ϵ���Թܿڡ�ʵ��ʱ�������е�Na2O2���䵽�Թ�b��ˮ�У���������������
A��U�ι��ڵĺ�ˮ��ɫ B���Թ�����Һ���
C������a������ D��U�ι��е�ˮλ��c�˸���d
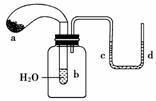
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ�������������Na2CO3��Na2SO4��CuSO4��CaCl2��NaCl��������ɡ�ijͬѧΪ�˼������ǣ���������ʵ�飺
�ٽ�������������ˮ����������ɫ����Һ��
���ڢ����õ���Һ�еμ�Ba(NO3)2��Һ���а�ɫ�������ɣ��������μ���������ȫ��
�۹��ˣ�Ȼ�������ð�ɫ�����ϼ������ϡ���ᣬ���������ܽ⡣
���ݸ�ͬѧʵ������ش�
��1��ԭ������п϶��� ���϶�û�� �����ܺ��� ��
��2��д������ʵ������У����ܷ�����Ӧ�����ӷ���ʽ��
��
��3��Ҫ������ܺ��е����ʿɲ��õķ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ж�0.2mo1/LNa2SO4��Һ��������ȷ����(NA��ʾ�����ӵ�������ֵ)�� ��
A. 1L��Һ�к�0.4NA��Na+ B.1L��Һ�к�Na+��SO42-����Ϊ0.8NA
C.2L��Һ�к���0.4NA��SO42- D. 2L��Һ��Na+�����ʵ���Ũ��Ϊ0.4mol/L
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com